
ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ

Спросите врача о рецепте на ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ

Инструкция по применению ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Введение
Инструкция: информация для пользователя
Фульвестрант Стада 250 мг раствор для инъекций в предварительно заполненном шприце EFG
Прочитайте внимательно всю инструкцию перед началом использования этого лекарства, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться перечитать ее.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
- Это лекарство было назначено только вам, и не следует давать его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции
- Что такое Фульвестрант Стада и для чего он используется
- Что вам нужно знать перед началом использования Фульвестранта Стада
- Как использовать Фульвестрант Стада
- Возможные побочные эффекты
- Хранение Фульвестранта Стада
- Содержание упаковки и дополнительная информация
1. Что такое Фульвестрант Стада и для чего он используется
Фульвестрант Стада содержит активное вещество фульвестрант, которое относится к группе блокаторов эстрогена.
Эстрогены, тип женских половых гормонов, могут в некоторых случаях быть вовлечены в развитие рака молочной железы.
Фульвестрант используется:
- один, для лечения женщин в постменопаузе с типом рака молочной железы, называемым раком молочной железы с положительным рецептором эстрогена, который находится в локально продвинутой или метастатической форме, или
- в комбинации с палбоциклибом для лечения женщин с типом рака молочной железы, называемым раком молочной железы с положительным гормональным рецептором, раком молочной железы с отрицательным рецептором 2 фактора роста эпидермиса человека, который находится в локально продвинутой или метастатической форме. Женщины, которые не достигли менопаузы, также будут лечены препаратом, называемым агонистом гормона, выделяющего гормон лютеинизации (LHRH).
Фульвестрант может быть введен в комбинации с палбоциклибом. Важно, чтобы вы также прочитали инструкцию к палбоциклибу. Если у вас есть какие-либо вопросы о палбоциклибе, проконсультируйтесь с вашим врачом.
2. Что вам нужно знать перед началом использования Фульвестранта Стада
Не используйте Фульвестрант Стада
- если вы аллергичны к фульвестранту или к любому другому компоненту этого лекарства (указанному в разделе 6)
- если вы беременны или кормите грудью
- если у вас есть тяжелые проблемы с печенью.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом, фармацевтом или медсестрой перед началом использования фульвестранта, если что-либо из этого относится к вам:
- проблемы с почками или печенью
- низкий уровень тромбоцитов (которые помогают в свертывании крови) или кровотечения
- предыдущие проблемы с тромбами
- остеопороз (потеря плотности костей)
- алкоголизм.
Дети и подростки
Фульвестрант не показан для использования у детей и подростков моложе 18 лет.
Использование Фульвестранта Стада с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство.
В частности, вы должны сказать вашему врачу, если вы используете антикоагулянты (лекарства для предотвращения тромбов).
Беременность и лактация
Беременность
Не следует использовать фульвестрант, если вы беременны. Если вы можете забеременеть, вы должны использовать эффективный метод контрацепции во время лечения фульвестрантом и в течение двух лет после последней дозы.
Лактация
Не следует кормить грудью во время лечения фульвестрантом.
Вождение и использование машин
Не ожидается, что Фульвестрант Стада повлияет на вашу способность управлять транспортными средствами или использовать машины. Однако, если вы чувствуете усталость после лечения, не управляйте транспортными средствами и не используйте машины.
Использование в спорте
Пациентам следует предупредить, что это лекарство содержит фульвестрант, который может дать положительный результат в тестах на допинг.
Фульвестрант Стада содержит этанол
Это лекарство содержит 1000 мг этанола в каждой дозе 500 мг фульвестранта. Количество этанола в каждой дозе 500 мг этого лекарства эквивалентно 25 мл пива или 10 мл вина.
Маловероятно, что количество этанола в этом лекарстве окажет влияние на взрослых.
Этанол, присутствующий в этом лекарстве, может изменить эффекты других лекарств. Поговорите с вашим врачом или фармацевтом, если вы принимаете другие лекарства.
Если вы страдаете алкоголизмом, проконсультируйтесь с вашим врачом или фармацевтом перед приемом этого лекарства.
Фульвестрант Стада содержит бензиловый спирт
Это лекарство содержит 1000 мг бензилового спирта в каждой дозе 500 мг фульвестранта. Бензиловый спирт может вызвать аллергические реакции.
Проконсультируйтесь с вашим врачом или фармацевтом, если у вас есть заболевания печени или почек. Это связано с тем, что могут накапливаться большие количества бензилового спирта в организме и вызывать побочные эффекты (метаболический ацидоз).
Фульвестрант Стада содержит бензоат бензила
Это лекарство содержит 1500 мг бензоата бензила в каждой дозе 500 мг фульвестранта.
3. Как использовать Фульвестрант Стада
Следуйте точно инструкциям по введению этого лекарства, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Рекомендуемая доза составляет 500 мг фульвестранта (две инъекции по 250 мг/5 мл) один раз в месяц с дополнительной дозой 500 мг, введенной через 2 недели после начальной дозы.
Ваш врач или медсестра введет фульвестрант путем медленной внутримышечной инъекции в каждый из ваших ягодиц.
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, спросите вашего врача, фармацевта или медсестру.
4. Возможные побочные эффекты
Как и все лекарства, фульвестрант может вызывать побочные эффекты, хотя не все люди испытывают их.
Вам может потребоваться срочная медицинская помощь, если вы испытываете любой из следующих побочных эффектов:
• Аллергические реакции (гиперчувствительность), включая отек лица, губ, языка и/или горла, которые могут быть симптомами анафилактических реакций.
• Тромбоэмболия (повышенный риск тромбов)*
• Воспаление печени (гепатит)
• Печеночная недостаточность
Немедленно сообщите вашему врачу, фармацевту или медсестре, если вы заметите любой из следующих побочных эффектов:
Очень частые побочные эффекты(может повлиять на более 1 из 10 человек)
- реакции в месте инъекции, такие как боль и/или воспаление
- анормальные уровни печеночных ферментов (в анализе крови)*
- тошнота (чувство дискомфорта)
- слабость, усталость*
- суставная и мышечная боль
- приливы
- кожная сыпь.
- аллергические реакции (гиперчувствительность), включая отек лица, губ, языка и/или горла
Все остальные побочные эффекты:
Частые побочные эффекты(может повлиять на до 1 из 10 человек)
- головная боль
- рвота, диарея или потеря аппетита*
- инфекции мочевыводящих путей
- боль в спине*
- повышение билирубина (пигмента желчи, производимого печенью)
- тромбоэмболия (повышенный риск тромбов)*
- пониженные уровни тромбоцитов (тромбоцитопения)
- вагинальное кровотечение
- боль в нижней части спины, отражающаяся на одной стороне ноги (ишиас)
- внезапная слабость, онемение, покалывание или потеря движения в ноге, особенно на одной стороне тела, внезапные проблемы с ходьбой или равновесием (периферическая нейропатия).
Редкие побочные эффекты(может повлиять на до 1 из 100 человек)
- густой, белый вагинальный секрет и кандидоз (инфекция)
- гематома и кровотечение в месте инъекции
- повышение гамма-ГТ, печеночного фермента, определяемого в анализе крови
- воспаление печени (гепатит)
- печеночная недостаточность
- онемение, покалывание и боль
- анафилактические реакции
- Включает побочные эффекты, для которых невозможно оценить точную роль фульвестранта из-за основного заболевания.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, сообщите вашему врачу, фармацевту или медсестре, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Вы также можете сообщить об этом напрямую через систему фармакологического надзора за лекарствами для человеческого использования: www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более подробной информации о безопасности этого лекарства.
5. Хранение Фульвестранта Стада
Храните это лекарство в недоступном для детей месте.
Храните и перевозите в холодильнике (при температуре между 2°C и 8°C).
Храните предварительно заполненный шприц в оригинальной упаковке, чтобы защитить его от света.
Отклонения температуры от диапазона между 2°C и 8°C должны быть контролируемы. Это включает в себя избежание хранения при температурах выше 30°C и то, что оно не превышает период 28 дней, в течение которого средняя температура хранения лекарства ниже 25°C (но выше диапазона между 2°C и 8°C). После отклонений температуры лекарство должно быть немедленно возвращено к рекомендуемым условиям хранения (хранить и перевозить в холодильнике при температуре между 2°C и 8°C). Отклонения температуры имеют накопительный эффект на качество лекарства, не должны превышать период 28 дней сверх срока годности фульвестранта. Воздействие температур ниже 2°C не повредит лекарство, если только оно не хранится при температурах ниже -20°C.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке или на этикетках шприцев после аббревиатуры CAD. Дата истечения срока годности - последний день месяца, указанного.
Ваш медицинский специалист будет отвечать за правильное хранение, использование и утилизацию Фульвестранта Стада.
Это лекарство может представлять риск для водной среды. Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт SIGRE аптеки. Спросите вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Фульвестранта Стада
- Активное вещество - фульвестрант. Каждый предварительно заполненный шприц (5 мл) содержит 250 мг фульвестранта. Каждый мл раствора содержит 50 мг фульвестранта.
- Другие компоненты (вспомогательные вещества) - этанол (96%), бензиловый спирт, бензоат бензила и рафинированное касторовое масло.
Внешний вид продукта и содержание упаковки
Фульвестрант Стада - прозрачный раствор, от бесцветного до желтого, практически свободный от видимых частиц, масляный и вязкий, содержащийся в предварительно заполненном шприце из стекла. Каждый шприц содержит 5 мл инъекционного раствора.
Фульвестрант Стада выпускается в трех форматах упаковки:
- Коробка из картона с блистером, содержащим предварительно заполненный шприц, стерильную гиподермическую иглу (BD SafetyGlide) и инструкцию.
или
- Коробка из картона с двумя блистерами, содержащими по одному предварительно заполненному шприцу, две стерильные гиподермические иглы (BD SafetyGlide) и инструкцию.
или
- Коробка из картона с шестью блистерами, содержащими по одному предварительно заполненному шприцу, шесть стерильных гиподермических игл (BD SafetyGlide) и инструкцию.
Возможно, только некоторые размеры упаковки будут продаваться.
Владелец разрешения на маркетинг
Лаборатория STADA, С.Л.
Фредерик Момпу, 5
08960 Сант Жуст Десверн (Барселона)
Испания
Производитель
S.C. Rompharm Company S.R.L.
Улица Эройлор, 1А,
Отопени 075100
Румыния
или
STADA Arzneimittel AG
Стадастр. 2-18, Дортельвиль
Д-61118 Бад Вильбель
Гессен
Германия
или
STADApharm GmbH
Феодор-Линен-Штр. 35
Д-30625 Ганновер
Германия
или
STADA Arzneimittel GmbH
Гешефтсаншадресс -
Мутгассе 36/2 Дёблинг
А-1190 Вена
Австрия
Дата последнего пересмотра этой инструкции:декабрь 2020
Подробная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям для человека (AEMPS) http://www.aemps.gob.es/.
---------------------------------------------------------------------------------------------------------------------
Эта информация предназначена только для медицинских специалистов:
Фульвестрант Стада должен быть введен с использованием двух предварительно заполненных шприцев, см. раздел 3.
Инструкции по введению
Ввести инъекцию в соответствии с местными рекомендациями по внутримышечному введению больших объемов.
ПРИМЕЧАНИЕ: Из-за близости седалищного нерва следует быть осторожным, если фульвестрант вводится в дорсоглютейную зону (см. раздел 4.4).
Предостережения: Не стерилизуйте в автоклаве иглу с системой безопасности (Игла Hipodermic Protegida “BD SafetyGlide”) перед ее использованием.
Руки должны оставаться позади иглы во все время ее использования и утилизации.
Для каждой из двух шприцев:
- Удалите стеклянный корпус шприца из упаковки (блистера) и проверьте, что он не поврежден.
- Откройте внешнюю упаковку иглы с системой безопасности (“SafetyGlide”).
- Перед введением необходимо визуально осмотреть парентеральные растворы на наличие частиц и изменений цвета.
- Держите шприц в вертикальном положении.
- Другой рукой возьмите пробку и осторожно поверните ее и удалите. Чтобы сохранить стерильность, не трогайте кончик шприца (см. Фигуру 1).
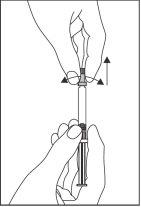
Фигура 1
?? Присоедините иглу с системой безопасности к соединителю “Luer-Lok” и завинтите ее до тех пор, пока она не будет надежно закреплена (см. Фигуру 2).
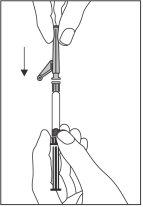
Фигура 2
- Проверьте, что игла присоединена к соединителю Luer-Lock, прежде чем перестать держать его в вертикальном положении.
- Аккуратно потяните за защитный колпачок иглы в прямой линии, чтобы не повредить кончик иглы.
- Перенесите заполненный шприц в место введения.
- Удалите защитный колпачок иглы.
- Удалите излишки газа из шприца.
- Введение должно быть произведено медленно внутримышечно в ягодицы (зону ягодиц) (1-2 минуты/инъекция). Для большего комфорта положение иглы с бизелем вверх имеет ту же ориентацию, что и поднятая рука рычага (см. Фигуру 3).
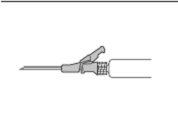
Фигура 3
?? После инъекции немедленно произведите один касание пальцем на руку рычага, чтобы активировать механизм защиты (см. Фигуру 4).
ПРИМЕЧАНИЕ: Активируйте на расстоянии от вашего тела и других. Слушайте щелчок и визуально подтверждайте, что кончик иглы полностью защищен.
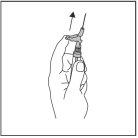
Фигура 4
Утилизация
Предварительно заполненные шприцы предназначены для одноразового использования.
Это лекарство может представлять риск для водной среды. Утилизация неиспользованного лекарства и всех материалов, которые вступали в контакт с ним, должна быть произведена в соответствии с местными правилами.

Сколько стоит ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Испании в 2025 году?
Средняя цена на ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в декабрь, 2025 года составляет около 225.7 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках225.7 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мг/5 млАктивное вещество: ФулвестрантПроизводитель: Bexal Farmaceutica S.A.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мгАктивное вещество: ФулвестрантПроизводитель: Ever Valinject GmbhТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 250 мгАктивное вещество: ФулвестрантПроизводитель: Astrazeneca AbТребуется рецепт
Аналоги ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Польша
Аналог ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ в Украина
Врачи онлайн по ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ФУЛВЕСТРАНТ СТАДА 250 мг РАСТВОР ДЛЯ ИНЪЕКЦИЙ В ПРЕДНАПОЛНЕННОМ ШПРИЦЕ – по решению врача и с учетом местных правил.












