
FRAGMIN 5.000 UI/0,2 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS

Cómo usar FRAGMIN 5.000 UI/0,2 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el usuario
FRAGMIN 5.000 UI/0,2 ml solución inyectable en jeringas precargadas
dalteparina sódica
Lea todo el prospecto detenidamente antes de empezar a utilizar el medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, o farmacéutico o personal de enfermería.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o personal de enfermería, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Fragmin y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fragmin
- Cómo usar Fragmin
- Posibles efectos adversos
- Conservación de Fragmin
- Contenido del envase e información adicional
1. Qué es Fragmin y para qué se utiliza
Fragmin pertenece al grupo de medicamentos llamados heparinas de bajo peso molecular.
Fragmin 5.000 UI/0,2 ml se utiliza en adultos a partir de 18 años de edad para:
- Profilaxis de la enfermedad tromboembólica en cirugía (para prevenir la trombosis venosa cuando usted se somete a una operación).
- Profilaxis de la enfermedad tromboembólica en pacientes no quirúrgicos inmovilizados cuya situación pueda definirse como de riesgo moderado o elevado (para prevenir la trombosis venosa cuando no va a ser sometido a una operación, pero se encuentra inmovilizado y presenta un riesgo importante de que se produzca trombosis).
- Tratamiento de la trombosis venosa profunda con o sin embolia pulmonar (para tratar coágulos de sangre ya existentes en las venas profundas -un tipo de vasos sanguíneos-).
- Prevención de los coágulos del sistema extracorpóreo durante la hemodiálisis y hemofiltración en los enfermos con insuficiencia renal crónica (para prevenir la formación de coágulos en el dializador durante la diálisis o la filtración de la sangre que se realiza en los pacientes que presentan una disminución de la capacidad de funcionamiento del riñón).
- Profilaxis de la enfermedad tromboembólica en pacientes con cáncer.
2. Qué necesita saber antes de empezar a usar Fragmin
No use Fragmin si:
- es alérgico (hipersensible) a la dalteparina sódica, a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6), a cualquier tipo de heparina (medicamentos que evitan la coagulación de la sangre) distinta a la dalteparina sódica o a productos derivados del cerdo
- padece úlcera gastroduodenal aguda, hemorragia cerebral u otro tipo de sangrados importantes
- presenta alteraciones graves de la coagulación
- presenta una enfermedad llamada endocarditis séptica aguda o subaguda (inflamación de una de las membranas del corazón debida a una infección)
- ha sufrido una operación en el sistema nervioso central, ojos u oídos, o si presenta traumatismos en estos órganos o sistema
- presenta una disminución del número de plaquetas (células presentes en la sangre que intervienen en la coagulación de la misma) y cuando se le realiza una prueba de agregación en presencia de dalteparina sódica, el resultado es positivo.
Si está en tratamiento con Fragmin no le podrán poner una anestesia epidural o espinal.
Advertencias y precauciones:
Consulte a su médico, farmacéutico o personal de enfermería antes de empezar a usar Fragmin si:
- Va a recibir inyecciones intramusculares de otros medicamentos por riesgo de hematomas.
- Presenta una disminución del número de plaquetas o defectos en las mismas. Su médico podrá solicitarle pruebas específicas para determinar la causa de este problema.
- Padece insuficiencia renal grave (disminución de la capacidad de funcionamiento del riñón) o insuficiencia hepática grave (disminución de la capacidad de funcionamiento del hígado).
- Tiene la tensión arterial elevada y no controlada.
- Padece trastornos en la retina (una parte del ojo) debidos a diabetes o a una tensión arterial elevada.
- Le han operado recientemente o presenta un riesgo elevado de hemorragia.
- Ha sufrido un infarto agudo de miocardio y está en tratamiento con este medicamento.
- Presenta riesgo de sufrir una elevación de los niveles de potasio en sangre por alguna enfermedad o por tomar determinados medicamentos. Su médico puede hacerle análisis para medir los niveles de potasio.
- Se le somete a anestesia epidural (en una de las membranas que rodean el cerebro y la médula espinal) o espinal (en la médula espinal) o a una punción lumbar, y se le administra heparina con fines de prevención; pueden aparecer muy raramente hematomas en estas zonas. Si usted sintiera dolor lumbar, entumecimiento, debilidad en las extremidades inferiores o algún trastorno en el funcionamiento del intestino o de la vejiga, informe inmediatamente a su médico.
- Tiene una prótesis valvular cardíaca, las dosis preventivas de Fragmin pueden no ser suficientes para evitar la trombosis valvular.
- Recibe tratamiento prolongado por enfermedad coronaria inestable, como previo a la revascularización, su médico puede reducirle la dosis de Fragmin.
- Es alérgico o sospecha que tiene una posible alergia al látex (goma natural) o si el capuchón de la aguja de las jeringas precargadas de Fragmin va a ser manipulado por alguien con una alergia conocida o posible al látex (goma natural). El capuchón de la aguja de las jeringas precargadas de Fragmin está hecho de látex (goma natural) que puede causar reacciones alérgicas graves en personas con alergia al látex (goma natural).
Considerando su estado y/o edad, su médico puede realizarle pruebas para controlar la actividad anticoagulante y evitar el riesgo de hemorragias o repetición de trombosis.
No debe intercambiarse Fragmin con otras heparinas no fraccionadas, heparinas de bajo peso molecular o polisacáridos sintéticos ya que su efecto puede no ser el mismo.
Niños y adolescentes:
Fragmin no se utiliza en recién nacidos menores de 1 mes de edad.
Uso de Fragmin con otros medicamentos:
Informe a su médico, farmacéutico o personal de enfermería si está utilizando, o ha utilizado recientemente otros medicamentos incluso los adquiridos sin receta médica.
Ciertos medicamentos pueden interaccionar con Fragmin 5.000 UI/0,2 ml; en estos casos puede resultar conveniente cambiar la dosis o interrumpir el tratamiento con alguno de los medicamentos.
El tratamiento trombolítico (que disuelve el coágulo) o algunos medicamentos que afectan a la coagulación de la sangre pueden aumentar el riesgo de hemorragia cuando se combinan con Fragmin:
- Aspirina (ácido acetilsalicílico).
- Inhibidores plaquetarios (se utilizan para disminuir la agregación plaquetaria y reducir el riesgo de coágulos sanguíneos).
- Trombolíticos (se utilizan para disolver los coágulos de sangre).
- Antiinflamatorios no esteroideos (AINEs) (medicamentos que se utilizan para tratar la inflamación).
- Antagonistas de la vitamina K y otros tipos de anticoagulantes.
- Dextrano (se utiliza en algunas lágrimas artificiales).
Embarazo y lactancia:
Consulte a su médico o farmacéutico antes de tomar o usar un medicamento, incluyendo Fragmin.
Si está usted embarazada, sólo debe usar este medicamento cuando sea claramente necesario aunque Fragmin no atraviesa la placenta.
No se recomienda su uso junto con la anestesia epidural. Informe a su médico si tiene una válvula cardiaca artificial.
Si está dando el pecho a su niño, informe a su médico; él valorará si el tratamiento con este medicamento es adecuado, ya que Fragmin pasa en pequeñas cantidades a la leche materna.
Conducción y uso de máquinas:
Este medicamento no altera la capacidad para conducir vehículos ni para usar maquinaria.
Fragmin contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por jeringa precargada; esto es, esencialmente “exento de sodio”.
3. Cómo usar Fragmin
Siga exactamente las instrucciones de administración de Fragmin 5.000 UI/0,2 ml indicadas por su médico. Consulte a su médico, farmacéutico o personal de enfermería si tiene dudas.
Recuerde tomar o usar su medicamento.
Su médico le indicará la dosis, el modo de empleo y la duración de su tratamiento con Fragmin.
Fragmin se administra por debajo de la piel (por vía subcutánea) para tratar los coágulos de sangre.
Si se está sometiendo a hemodiálisis o hemofiltración (procedimientos utilizados para purificar la sangre), Fragmin se le administrará en una vena o en el tubo de la máquina de diálisis.
Si a usted se le va a administrar Fragmin para prevenir la trombosis venosa cuando va a ser sometido a una operación, la dosis va a depender del tipo de operación a la que usted sea sometido.
Si usted va a ser sometido a una operación de cirugía general que comporte un riesgo moderado de trombosis, se le administrarán 2.500 UI (anti-Xa) mediante inyección aplicada por debajo de la piel, de 2-4 horas antes de la misma. Después de la operación, se le administrarán 2.500 UI una vez al día, durante el periodo de riesgo o hasta que usted pueda ya moverse.
Si usted va a ser sometido a una operación de cirugía oncológica u ortopédica que comporte un riesgo elevado de trombosis, se le administrarán 2.500 UI (anti-Xa) mediante inyección aplicada por debajo de la piel, de 2-4 horas antes de la misma. Doce horas después de la operación, se le administrarán de nuevo 2.500 UI (anti-Xa), y posteriormente 5.000 UI (anti-Xa) al día, una inyección de Fragmin 5.000 UI/0,2 ml o dos inyecciones de Fragmin 2.500 UI/0,2 ml, mientras dure el periodo de riesgo o hasta que usted pueda ya moverse.
Si a usted se le va a administrar Fragmin 5.000 UI/0,2 ml para prevenir la trombosis venosa cuando no se ha sometido a una operación, pero se encuentra inmovilizado, la dosis va a depender del riesgo que usted presente. Si el riesgo de trombosis es moderado, se le administrarán 2.500 UI una vez al día. Si es elevado, la dosis será de 5.000 UI, una inyección de Fragmin 5.000 UI/0,2 ml, al día. En ambos casos se le administrará este medicamento mientras dure el periodo de riesgo o hasta que usted pueda ya moverse.
Si a usted se le va a administrar Fragmin 5.000 UI/0,2 ml para tratar coágulos de sangre ya existentes en las venas profundas o para prevenir la formación de coágulos en el dializador durante la diálisis o la filtración de la sangre realizadas porque la capacidad de funcionamiento del riñón está disminuida, su médico le indicará con qué frecuencia se va a administrar este medicamento, así como la dosis a utilizar y la duración del tratamiento.
Las inyecciones de Fragmin normalmente le serán administradas por un profesional sanitario; se le aplicarán por debajo de la piel, en la zona anterior o posterior del abdomen y alternativamente en el lado derecho e izquierdo.
Uso en niños y adolescentes
Tratamiento de los coágulos de sangre en las venas (tromboembolia venosa [TEV] sintomática)
Las dosis recomendadas dependen del peso corporal del niño y del grupo de edad. El médico realizará el cálculo. El médico le aconsejará sobre la dosis individualizada de Fragmin de acuerdo con estos criterios. No cambie la dosis ni la pauta de tratamiento sin consultar antes con su médico.
Este medicamento no es adecuado para su uso en la población pediátrica debido a que las jeringas precargadas no permiten ajustar la dosis en base al peso corporal del niño. Se recomienda utilizar la presentación 2.500 UI/ml solución inyectable en viales de 4 mL.
El efecto de Fragmin se controlará después de la dosis inicial y el posterior ajuste de la dosis mediante un análisis de sangre.
Cómo inyectar Fragmin
Fragmin se administra mediante una inyección debajo de la piel (por vía subcutánea). Esta sección del prospecto explica cómo debe administrarse Fragmin. Debe seguir estas instrucciones únicamente después de haber sido entrenado por su médico. Si no está seguro de qué hacer, hable con su médico de inmediato. Debe inyectarse (o administrar) la dosis de Fragmin en los horarios recomendados por su médico.
Si es necesario realizar una dilución antes de administrar Fragmin, la debe realizar un profesional sanitario. Debe seguir las instrucciones de su médico sobre cómo y cuándo inyectar el medicamento diluido que se le proporcione.
Por favor, siga los pasos que se explican a continuación
Paso1: La forma de preparar la jeringa para la inyección dependerá de la presentación específica de Fragmin que se vaya a utilizar.
El sistema de seguridad Needle-Trapestá especialmente diseñado para ayudar a prevenir accidentes con las agujas después del uso correcto de Fragmin. Consiste en un dispositivo de seguridad de plástico acoplado a la etiqueta de la jeringa. Se utiliza para evitar pinchazos accidentales tras la correcta inyección de Fragmin. El dispositivo de protección para la aguja (Needle-Trap) consiste en una lengüeta de plástico (pinza) que se encuentra alineada en paralelo con la aguja, firmemente acoplada a la etiqueta del cilindro de la jeringa.
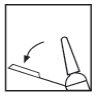
Se requieren las siguientes acciones para activar el sistema de seguridad: Levante la jeringa, sujete la punta del bloqueador de plástico de la aguja y dóblela alejándola del protector (ver Figura 1).
Figura 1
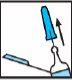
Retire el capuchón de goma gris tirando de él hacia afuera (ver Figura 2).
Figura 2
Notará una burbuja de aire en la jeringa. Esto está previsto y puede ignorarlo. Es importante no presionar el émbolo todavía, ya que se puede perder parte del medicamento. La burbuja de aire de las jeringas desechables no se debe expulsar antes de la inyección, ya que esto puede provocar la pérdida de medicamento y, por lo tanto, una reducción de la dosis.
Ahora está listo para administrar la inyección. Continúe con el paso 2.
Paso2: Elección y preparación de la zona de inyección subcutánea
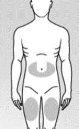
Elija uno de los sitios de inyección recomendados a continuación (ver las zonas sombreadas en la Figura 3):
- Una zona en forma de “U” alrededor del ombligo.
- Cara central de los muslos.
Figura 3
- Utilice un sitio de inyección diferente cada vez que se administre una dosis.
- No administre la inyección en zonas donde la piel esté dolorida, amoratada, enrojecida o dura. Evite las zonas con cicatrices.
- Si usted o el niño tienen psoriasis, no administre la inyección directamente en ninguna placa de piel elevada, gruesa, roja o escamosa (“lesiones cutáneas de psoriasis”).
- Lávese y séquese las manos.
- Limpie el sitio de la inyección con una toallita impregnada en alcohol nueva, con movimientos circulares. Deje que la piel se seque completamente. No vuelva a tocar esta zona antes de administrar la inyección.
Paso3: Adoptar la postura correcta
Usted o su hijo deben estar sentados o acostados para la administración de la inyección subcutánea. Si va a autoinyectarse el medicamento, siéntese en una posición cómoda en la que pueda ver su vientre (ver Figura 4).
Figura 4
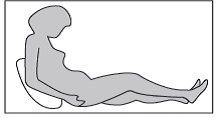
Paso4:
Con el pulgar y el índice, levante un pliegue de piel con una mano. Con la otra mano, sujete la jeringa como si fuera un lápiz. Este será el sitio de la inyección.
Paso5:
Si está inyectando Fragmin a un adulto o a usted mismo,sujete la jeringa sobre el pliegue de piel manteniéndola en ángulo recto (es decir, verticalmente como en el diagrama y no en ángulo). Introduzca la aguja en la piel hasta que esté completamente dentro (ver Figura 5).
Figura 5
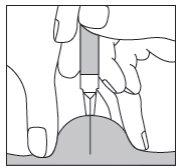
Si va a inyectar Fragmin a un niño,introduzca la aguja en la piel hasta el fondo con un movimiento pequeño y rápido, en un ángulo de entre 45° y 90° (ver Figura 6).
Figura 6
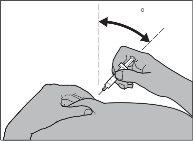
Paso 6:
Empuje el émbolo completamente hacia abajo a una velocidad lenta y constante para administrar la dosis correcta. Siga pellizcando el pliegue de piel mientras está administrando la inyección y luego suelte el pliegue de piel y saque la aguja.
Si hay alguna exudación de sangre en el sitio de la inyección, aplique una presión suave. No frote el sitio de la inyección, ya que esto puede provocar hematomas.
Presione el sitio de la inyección con una torunda de algodón durante 10 segundos. Puede producirse un ligero sangrado. No frote el sitio de la inyección. Puede colocar un vendaje sobre el sitio de la inyección.
Paso7: si su jeringa tiene un dispositivo de protecciónNeedle-Trap, actívelo
Coloque el bloqueador de plástico contra una superficie dura y estable y con una mano gire el cilindro de la jeringa hacia arriba contra la aguja, forzando la aguja hacia el bloqueador hasta que encaje (ver Figura 7).
Continúe doblando la aguja hasta que la jeringa exceda un ángulo de 45 grados con la superficie plana para inutilizarla de forma permanente (ver Figura 8).
(ver Figura 7) (ver Figura 8)
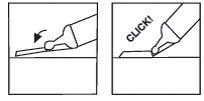
Paso8:
Deseche la jeringa y la aguja en un recipiente para objetos punzo-cortantes. Mantenga su recipiente para objetos punzo-cortantes fuera del alcance de otras personas. Cuando el recipiente para objetos punzo-cortantes esté casi lleno, deséchelo según las instrucciones o hable con su médico o enfermero.
Si usted usa más Fragmin del que debe
Si usted ha usado más Fragmin de lo que debe, consulte inmediatamente a su médico o farmacéutico, acuda al hospital más cercano o consulte al Servicio de Información Toxicológica, teléfono 91 562 04 20.
Si olvidó usar Fragmin
Consulte inmediatamente a su médico o farmacéutico.
No administrar una dosis doble para compensar las dosis olvidadas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Fragmin puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos observados de manera frecuente (pueden afectar hasta uno de cada 10 pacientes):
- Dolor y aparición de cardenales en el lugar de la inyección
- Disminución reversible del número de plaquetas de la sangre no mediada por mecanismos inmunitarios (tipo 1)
- Sangrado en cualquier punto que a veces ha sido mortal
- Aumento temporal de las enzimas del hígado
Efectos adversos raros (pueden afectar hasta uno de cada 1.000 pacientes):
- Caída del pelo, muerte de las células de la piel
Efectos adversos de frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- Disminución del número de plaquetas en la sangre mediada por mecanismos inmunitarios inducidos por la heparina (tipo 2)
- Reacciones alérgicas graves
- Sangrado localizado en el interior del cráneo, en el interior del abdomen o en otros lugares, a veces mortal
- Erupción
- Acumulación de sangre en el interior del cráneo o de la columna vertebral (hematoma epidural o espinal)
- Elevación de los niveles de potasio en sangre
- Osteoporosis (porosidad en los huesos)
Se espera que los efectos adversos en niños sean los mismos que los de los adultos. No obstante, se dispone de poca información sobre los posibles efectos adversos con el uso prolongado en niños.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento
5. Conservación de Fragmin
Mantener fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30ºC.
No utilice Fragmin después de la fecha de caducidad (CAD) que aparece en el embalaje y cartón exterior. La fecha de caducidad es el último día del mes que se indica.
No utilice Fragmin si presenta partículas o si aparece decoloración.
Los medicamentos no se deben tirar a los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente
6. Contenido del envase e información adicional
Composición de Fragmin 5.000 UI/0,2 ml:
El principio activo de Fragmin 5.000 UI/0,2 ml es la dalteparina sódica. Cada mililitro de solución contiene 25.000 UI (anti-Xa) de dalteparina sódica. El contenido total por envase es de 5.000 UI (anti-Xa).
Los demás componentes son: hidróxido de sodio, ácido clorhídrico y agua para preparaciones inyectables.
Aspecto de Fragmin 5.000 UI/0,2 ml y contenido del envase:
Jeringas precargadas con dispositivo de protección para la aguja: solución inyectable de administración subcutánea en jeringas precargadas con 5.000 UI (anti-Xa)/0,2 ml en envases con 10 y 25 jeringas precargadas.
Titular de la Autorización de Comercialización:
Pfizer, S.L.
Avda. de Europa 20-B. Parque Empresarial La Moraleja.
28108 Alcobendas (Madrid) España
Responsable de la Fabricación:
Catalent France Limoges S.A.S.
Z.I. Nord
53, Rue de Dion Bouton
87280 Limoges (Francia)
o
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Bélgica
Información adicional para el profesional sanitario/usuario:
Fragmin 5.000 UI/0,2 ml es compatible con soluciones de cloruro sódico isotónicas (9 mg/ml) o de glucosa (50 mg/ml), tanto en frascos de vidrio como en envases de plástico.
No se ha investigado la compatibilidad entre este medicamento y otros productos.
Este prospecto ha sido aprobado en junio 2025
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es
- País de registro
- Precio medio en farmacia46.58 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FRAGMIN 5.000 UI/0,2 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADASForma farmacéutica: INYECTABLE, 10.000 UI dalterapina sodica/0,4 mlPrincipio activo: dalteparinFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 10.000 UI/mlPrincipio activo: dalteparinFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 12.500 UI dalterapina sodica/0,5 mlPrincipio activo: dalteparinFabricante: Pfizer S.L.Requiere receta
Médicos online para FRAGMIN 5.000 UI/0,2 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FRAGMIN 5.000 UI/0,2 ml SOLUCION INYECTABLE EN JERINGAS PRECARGADAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















