
TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose

Pergunte a um médico sobre a prescrição de TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose

Como usar TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose
Introdução
Prospecto: Informação para o paciente
Taptiqom 15 microgramas/ml + 5 mg/ml colírio em solução em embalagem unidose
Tafluprost / Timolol
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico, farmacêutico ou enfermeiro.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver seção 4.
Conteúdo do prospecto
- O que é Taptiqom e para que é utilizado
- O que precisa saber antes de começar a usar Taptiqom
- Como usar Taptiqom
- Possíveis efeitos adversos
- Conservação de Taptiqom
- Conteúdo do embalagem e informações adicionais
1. O que é Taptiqom e para que é utilizado
Que tipo de medicamentoé e como actua?
Taptiqom colírio em solução contém tafluprost e timolol. Tafluprost é um medicamento do grupo chamado análogos da prostaglandina e timolol pertence ao grupo de medicamentos chamados betabloqueantes. Tafluprost e timolol actuam conjuntamente e reduzem a pressão do olho. Taptiqom é utilizado quando a pressão do olho é demasiado alta.
Para que serve o seu medicamento?
Taptiqom é utilizado para tratar um tipo de glaucoma chamado glaucoma de ângulo aberto, uma afecção chamada também hipertensão ocular em adultos. Ambas situações relacionam-se com um aumento da pressão no olho e ocasionalmente podem afetar a sua visão.
2. O que precisa saber antes de começar a usar Taptiqom
Não use Taptiqom:
- se é alérgico a tafluprost, timolol, os betabloqueantes, ou a qualquer um dos outros componentes deste medicamento (incluídos na seção 6).
- se tem ou teve anteriormente problemas respiratórios como asma, bronquite obstructiva crónica grave (enfermidade pulmonar grave que pode provocar silvos, dificuldade respiratória ou tosse prolongada).
- se tem o ritmo cardíaco lento, insuficiência cardíaca ou distúrbios do ritmo cardíaco (pulso cardíaco irregular).
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Taptiqom.
Antes de utilizar este medicamento informe o seu médico se tem ou teve:
- enfermidade cardíaca (os sintomas podem incluir dor ou compressão torácicos, insuficiência respiratória ou asfixia), insuficiência cardíaca ou hipotensão arterial
- distúrbios do ritmo cardíaco, como latidos lentos
- problemas respiratórios, asma ou enfermidade pulmonar obstructiva crónica
- ma circulação sanguínea (como enfermidade de Raynaud ou síndrome de Raynaud)
- diabetes, porque o timolol pode ocultar os sinais e sintomas de hipoglicemia (níveis baixos de açúcar)
- hiperactividade da glândula tireoide, porque o timolol pode ocultar os sinais e sintomas de enfermidades tireoides
- qualquer alergia ou reação anafiláctica
- miastenia grave (enfermidade grave que provoca debilidade muscular)
- outras enfermidades oculares, como enfermidade da córnea (o tecido transparente que cobre a parte frontal do olho) ou uma enfermidade que precise cirurgia ocular.
Informe o seu médico se tem
- problemas de rim
- problemas de fígado
Tenha em contaque Taptiqom pode ter os efeitos seguintes e que alguns podem ser permanentes:
- Taptiqom pode aumentar o comprimento, espessura, cor ou densidade das suas pestanas e provocar um crescimento anormal de pelo nas pálpebras.
- Taptiqom pode provocar o escurecimento da pele ao redor dos olhos. Seque o excesso de solução da pele. Assim reduzirá o risco de escurecimento da pele.
- Taptiqom pode alterar a cor do seu íris (a parte colorida do seu olho). Se utilizar Taptiqom em um só olho, a cor do olho tratado pode ficar permanentemente diferente da cor do outro olho.
- Taptiqom pode causar crescimento de pelo em zonas onde a solução entra em contacto com a superfície da pele repetidamente.
Se vai ser submetido a uma operação, avise o seu médico de que está utilizando Taptiqom, porque o timolol pode alterar os efeitos de alguns medicamentos utilizados durante a anestesia.
Crianças e adolescentes
Taptiqom não é recomendado para crianças e adolescentes menores de 18 anos devido à falta de dados sobre segurança e eficácia nesta faixa etária.
Uso de Taptiqom com outros medicamentos
Comunique ao seu médico ou farmacêutico se está tomando, tomou recentemente ou acredita que possa ter que tomar qualquer outro medicamento.
Taptiqom pode afetar, ou ser afetado por, outros medicamentos que está tomando.
Em particular, informe o seu médico se usa/toma ou prevê usar/tomar:
- outros colírios para o tratamento do glaucoma
- medicamentos para reduzir a pressão sanguínea
- medicamentos para o coração
- medicamentos para o tratamento da diabetes
- quinidina (utilizada para tratar problemas cardíacos e alguns tipos de malária)
- antidepressivos conhecidos como fluoxetina ou paroxetina
Se utilizar outros medicamentos no olho, deixe passar como mínimo cinco minutos entre a instilação de Taptiqom e a do outro medicamento.
Lentes de contacto
Retire as lentes de contacto antes de administrar as gotas e espere como mínimo quinze minutos antes de voltar a colocá-las.
Gravidez, lactação e fertilidade
Se é uma mulher que pode engravidar, deve utilizar um método anticonceptivo eficaz durante o tratamento com Taptiqom. Não utilize Taptiqom se está grávida. Não utilize Taptiqom se está amamentando. Consulte o seu médico.
Condução e uso de máquinas
Alguns efeitos adversos associados a Taptiqom, como a visão borrosa, podem afetar a sua capacidade para conduzir veículos ou operar máquinas. Não conduza nem opere máquinas até que se sinta bem e a sua visão seja clara.
Taptiqom contém tampão fosfato
Este medicamento contém aproximadamente 0,04 mg de fosfatos em cada gota, equivalente a 1,3 mg/ml. Se sofre de dano grave na córnea (a camada transparente da parte frontal do olho) o tratamento com fosfatos, em casos muito raros, pode provocar visão borrosa por acumulação de cálcio.
3. Como usar Taptiqom
Siga exactamente as instruções de administração deste medicamento indicadas pelo seu médico ou farmacêutico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
A dose recomendada é de uma gota diária de Taptiqom no olho ou olhos afectados. Não instile mais gotas nem o faça mais frequentemente do que o indicado pelo seu médico. Se o fizer, Taptiqom poderá perder eficácia. Use Taptiqom em ambos os olhos apenas se o seu médico o tiver indicado. Elimine o embalagem aberto e o conteúdo restante imediatamente após utilizá-lo.
Para uso apenas como colírio. Não ingerir.
Nãodeixe que o embalagem unidose toque o olho nem a zona circundante. Poderia danificar o olho. Também poderia contaminar-se com bactérias que poderiam provocar infecções oculares que, por sua vez, poderiam provocar danos no olho, incluindo a perda da visão. Para evitar a possível contaminação do embalagem unidose, evite que a ponta do mesmo toque qualquer superfície.
Instruções de uso:
Quando abrir uma bolsa nova:
Não utilize o embalagem unidose se a bolsa estiver rasgada. Abra a bolsa rasgando ao longo da linha pontilhada. Escreva, no espaço da bolsa reservado a este efeito, a data em que abriu a bolsa.
Cada vez que use Taptiqom:
- Lave as mãos.
- Retire a tira de embalagens da bolsa.
- Desprenda um embalagem unidose da tira.
- Volte a colocar o resto da tira na bolsa e dobre a borda para fechá-la.
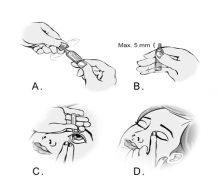
- Para abrir o embalagem, quebre a pestaña girando-a (figura A).
- Segure o embalagem com os dedos polegar e indicador. Certifique-se de que a ponta do embalagem não sobressaia mais de 5 mm do bordo do seu dedo indicador (figura B).
- Incline a cabeça para trás ou deite-se. Coloque a mão na testa. O seu indicador deve estar alinhado com a sobrancelha ou descansar sobre o bridge do nariz. Olhe para cima. Pressione para baixo a pálpebra anterior com a outra mão. Não deixe que nenhuma parte do embalagem toque o olho nem a zona circundante. Pressione suavemente o embalagem para que caia uma gota no espaço situado entre a pálpebra e o olho (figura C).
- Feche o olho e pressione a esquina interior do mesmo com o dedo durante uns dois minutos. Assim ajuda a impedir que a gota se vá pelo ducto lacrimal (figura D).
- Seque o excesso de solução da pele ao redor do olho.
Se a gota cair fora do olho,tente novamente.
Se o seu médico lhe disse que se aplique gotas em ambos os olhos, repita os passos 7 a 9 no outro olho. O conteúdo de um embalagem unidose é suficiente para ambos os olhos. Elimine o embalagem aberto e o conteúdo restante imediatamente após utilizá-lo.
Se usar outros medicamentos no olho,deixe passar como mínimo cinco minutos entre a aplicação de Taptiqom e a do outro medicamento.
Se usar mais Taptiqom do que deve, pode sentir-se mareado ou ter dor de cabeça, molestias cardíacas ou molestias respiratórias. Se for necessário, consulte um médico.
Se engolir acidentalmente o medicamento,consulte um médico.
Também pode ligar para o Serviço de Informação Toxicológica, telefone 91 562 04 20, indicando o medicamento e a quantidade tomada.
Se esquecer de usar Taptiqom,coloque uma gota assim que se lembrar e volte à rotina normal. No entanto, se está próxima a hora da dose seguinte, salte a dose esquecida. Não tome uma dose dupla para compensar as doses esquecidas.
Não deixe de utilizar Taptiqom sem consultar o seu médico. Se interromper o tratamento com Taptiqom, a pressão do olho voltará a aumentar. Isso poderia provocar um dano permanente no olho. Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico, farmacêutico ou enfermeiro.
4. Possíveis efeitos adversos
Como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora não todas as pessoas os sofram. A maioria dos efeitos adversos não é grave.
Normalmente poderá continuar a usar as gotas, excepto se os efeitos forem graves. Em caso de dúvida, consulte um médico ou farmacêutico.
Os efeitos adversos conhecidos do uso de Taptiqom são:
Efeitos adversos frequentes
Os efeitos seguintes podem afetar até 1 de cada 10 pessoas:
Distúrbios oculares
Coceira nos olhos. Irritação nos olhos. Dor nos olhos. Vermelhidão nos olhos. Alterações no comprimento, espessura e densidade das pestanas. Sensação de corpos estranhos no olho. Descoloração das pestanas. Sensibilidade à luz. Visão borrosa.
Efeitos adversos pouco frequentes
Os efeitos seguintes podem afetar até 1 de cada 100 pessoas:
Distúrbios do sistema nervoso
Dor de cabeça.
Distúrbios oculares
Secura ocular. Vermelhidão das pálpebras. Pequenas zonas de inflamação em pontos na superfície do olho. Olhos lacrimejantes. Inchaço das pálpebras. Olhos cansados. Inflamação das pálpebras. Inflamação dentro do olho. Molestias no olho. Alergia ocular. Inflamação do olho. Sensação anormal no olho.
Os efeitos adversos adicionais seguintes foram observados nos medicamentos que compõem Taptiqom (tafluprost e timolol) e, portanto, poderiam aparecer ao utilizar Taptiqom:
Os efeitos secundários seguintes foram observados com tafluprost:
Distúrbios oculares
Redução da capacidade do olho para ver detalhes. Alteração da cor do íris (pode ser permanente). Alteração da cor da pele ao redor dos olhos. Inflamação das membranas da superfície do olho. Secreção ocular. Pigmentação das membranas da superfície do olho. Fólicos nas membranas da superfície do olho. Olho afundado. Irite/uveíte (inflamação da parte colorida do olho). Edema macular/edema macular cístico (inflamação da retina dentro do olho, levando ao pioramento da visão).
Distúrbios da pele
Crescimento anormal de pelo nas pálpebras.
Efeitos sobre o sistema respiratório
Pioramento do asma, insuficiência respiratória.
Os efeitos secundários seguintes foram observados com timolol:
Distúrbios do sistema imunológico
Reações alérgicas, incluindo inflamação sob a pele, urticária e erupções. Reação alérgica repentina e potencialmente mortal. Coceira.
Distúrbios do metabolismo e da nutrição
Hipoglicemia (diminuição dos níveis de açúcar).
Distúrbios psiquiátricos
Depressão. Distúrbios do sono. Pesadelos. Perda de memória. Nervosismo. Alucinações
Distúrbios do sistema nervoso
Tonturas. Fraqueza. Sensações incomuns (como formigamento e picadas). Aumento dos sinais e sintomas de miastenia grave (distúrbio muscular). Acidente vascular cerebral. Redução do fluxo sanguíneo do cérebro.
Distúrbios oculares
Inflamação da córnea. Redução da sensibilidade da córnea. Perturbações visuais, incluindo alterações refractivas (às vezes devido ao abandono da terapia miótica). Queda da pálpebra superior. Visão dupla. Visão borrosa e desprendimento da camada situada sob a retina, que contém os vasos sanguíneos, após uma cirurgia por filtração, o que pode provocar perturbações da vista. Erosão da córnea.
Distúrbios auditivos
Acúfenos (zumbidos nos ouvidos).
Distúrbios cardíacos
Latidos do coração lentos. Dor no peito. Palpitações. Edema (acumulação de líquido). Alterações do ritmo ou da velocidade dos latidos do coração. Insuficiência cardíaca congestiva (enfermidade cardíaca com dificuldade respiratória e inchaço de pés e pernas devido à acumulação de fluidos). Um tipo de distúrbio do ritmo cardíaco. Infarto do miocárdio. Falha cardíaca.
Distúrbios vasculares
Pressão arterial baixa. Claudicação. Fenómeno de Raynaud, mãos e pés frios.
Distúrbios respiratórios
Contração das vias respiratórias nos pulmões (especialmente em pacientes com uma enfermidade prévia). Dificuldade respiratória. Tosse.
Distúrbios gastrointestinais
Náuseas. Indigestão. Diarreia. Boca seca. Alterações do paladar. Dor abdominal. Vômitos.
Distúrbios da pele
Perda de cabelo. Erupção cutânea com aspecto branco prateado (erupção como a psoríase) ou pioramento da psoríase. Erupção cutânea.
Distúrbios musculares e esqueléticos
Dor muscular não provocada pelo exercício. Dor articular.
Distúrbios do sistema reprodutor e da mama
Enfermidade de La Peyronie (que pode provocar uma curvatura do pênis). Disfunção sexual. Redução da libido.
Distúrbios gerais
Fraqueza muscular/cansaço. Sede.
Comunicação de efeitos adversos
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico ou enfermeiro, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Também pode comunicá-los directamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https;//www.notificaram.es.
Ao comunicar efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Taptiqom
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize este medicamento após a data de validade que aparece no embalagem unidose, na bolsa e na caixa após "CAD" e “EXP”. A data de validade é o último dia do mês que se indica.
Guarde as bolsas de alumínio sem abrir na geladeira (entre 2 e 8 °C). Não abra a bolsa até que vá começar a utilizar o colírio, porque os embalagens não utilizados de uma bolsa aberta devem ser eliminados 28 dias após abrir a bolsa pela primeira vez.
Após abrir a bolsa de alumínio:
- Conservar os embalagens unidose na bolsa de alumínio original para protegê-los da luz.
- Conservar abaixo de 25 °C.
- Eliminar os embalagens unidose passados 28 dias desde a primeira abertura da bolsa de alumínio.
- Eliminar os embalagens unidose abertos com a solução restante imediatamente após utilizá-los.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os embalagens e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos embalagens e dos medicamentos que já não precisa. Dessa forma, ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Taptiqom
- Os princípios ativos são tafluprost e timolol. 1 ml de solução contém 15 microgramas de tafluprost e 5 mg de timolol.
- Os demais componentes são glicerol, fosfato disódico dodecahidratado, edetato disódico, polissorbato 80, ácido clorídrico ou hidróxido sódico (para ajustar o pH) e água para preparações injetáveis.
Aspecto do produto e conteúdo do envase
Taptiqom é um líquido (solução) transparente e incolor apresentado em envases unidose de plástico que contêm 0,3 ml de solução cada um. Os envases unidose estão contidos, de dez em dez, em uma bolsa. Taptiqom é fornecido em envases com 30 ou 90 envases unidose.
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finlândia
Responsável pela fabricação
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finlândia
Podem solicitar mais informações sobre este medicamento dirigindo-se ao representante local do título da autorização de comercialização:
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu e no Reino Unido (Irlanda do Norte) com os seguintes nomes:
Taptiqom:Alemanha, Áustria, Bélgica, Bulgária, Chipre, Croácia, Dinamarca, Eslováquia, Eslovênia, Espanha, Estônia, Finlândia, França, Grécia, Holanda, Hungria, Irlanda, Islândia, Letônia, Lituânia, Luxemburgo, Malta, Noruega, Polônia, Portugal, Reino Unido (Irlanda do Norte), República Checa, Romênia, Suécia
Loyada:Itália
Data da última revisão deste prospecto:junho 2022
A informação detalhada deste medicamento está disponível na página web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/

Quanto custa o TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose em Espanha em 2025?
O preço médio do TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose em dezembro de 2025 é de cerca de 26.09 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia26.09 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidoseForma farmacêutica: COLÍRIO, 10 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Novartis Europharm LimitedRequer receita médicaForma farmacêutica: COLÍRIO, 0,3 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Brill Pharma S.L.Requer receita médicaForma farmacêutica: COLÍRIO, 0,3 mg/ml + 5 mg/mlSubstância ativa: timolol, combinationsFabricante: Laboratorio Stada S.L.Requer receita médica
Alternativas a TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose em Polónia
Alternativa a TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose em Ukraine
Médicos online para TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de TAPTIQOM 15 microgramas/ml + 5 mg/ml colírio, solução em embalagens unidose – sujeita a avaliação médica e regras locais.














