How to use Mirena
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Mirena
52 mg, 20 micrograms/24 hours, intrauterine therapeutic system
Levonorgestrel
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Mirena and what is it used for
- 2. Important information before using Mirena
- 3. How to use Mirena
- 4. Possible side effects
- 5. How to store Mirena
- 6. Contents of the packaging and other information
1. What is Mirena and what is it used for
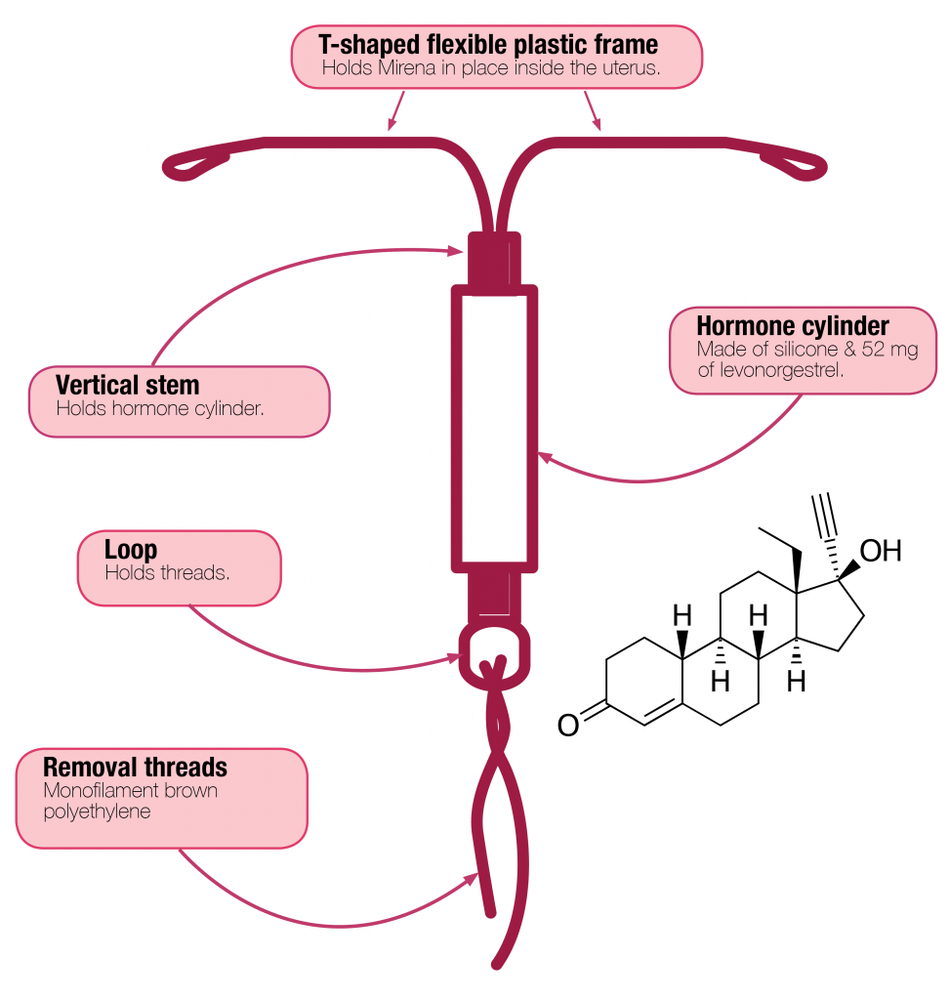
Mirena is an intrauterine therapeutic system in the shape of the letter "T", which, after insertion, releases the hormone - levonorgestrel into the uterus. The "T" shape ensures that the system fits into the uterus. The white system contains a reservoir with the active substance levonorgestrel. Two brown threads are attached to the loop at the bottom of the system, which are used to remove the system from the uterus.
Mirena is used to prevent pregnancy (contraceptive effect) and to treat excessive menstrual bleeding (of unknown origin).
Children and adolescents
Mirena is not indicated for use before the first menstrual bleeding (menarche).
2. Important information before using Mirena
General notes
Before using the Mirena system, the doctor will ask the patient a few questions about her health and the health of her close relatives.
This leaflet describes several situations in which it is necessary to remove the Mirena system or when the effectiveness of the system may be reduced. In such situations, you should either abstain from sexual intercourse or use additional non-hormonal contraceptive methods, such as a condom or another mechanical method. You should not use the calendar method or temperature measurement. They may prove to be ineffective, as Mirena affects monthly changes in body temperature and cervical mucus.
Mirena, like other hormonal contraceptives, does not protect against HIV (AIDS) or other sexually transmitted diseases.
Mirena should not be inserted if any of the following conditions are present:
- if the patient is allergic to levonorgestrel or any of the other components of this medicine (listed in section 6)
- pregnancy or suspected pregnancy
- tumors whose growth is dependent on the action of progesterone, such as breast cancer;
- existing or recurring inflammatory conditions of the pelvic organs (infections of the female reproductive organs)
- cervicitis
- infections of the lower genital tract
- postpartum endometritis
- infections of the uterus after miscarriage within the last 3 months
- conditions that promote the development of infection
- abnormal cervical cells
- cancer or suspected cancer of the cervix or uterus
- unexplained abnormal uterine bleeding
- abnormalities in the cervix or uterus, including uterine fibroids, if they deform the uterine cavity
- active liver disease or liver tumor.
Warnings and precautions
Before starting to use the Mirena system, you should consult a doctor.
Consult a doctor who will decide whether to remove the Mirena system or not if any of the following conditions occur for the first time while using the Mirena system:
- migraine, asymmetric visual field defects or other symptoms that may be signs of transient cerebral ischemia (temporary blockage of blood flow to the brain)
- severe headache
- jaundice (yellowing of the skin, whites of the eyes, and/or nails)
- significant increase in blood pressure
- severe arterial disease, such as stroke or heart attack
- acute venous thromboembolic disease.
The Mirena system should be used with caution in women with congenital heart defects or heart valve defects that increase the risk of endocarditis.
In women with diabetes who use the Mirena system, blood glucose levels should be monitored.
Irregular bleeding may mask some symptoms and signs of endometrial polyps or cancer, and in these cases, diagnostic tests should be considered.
Medical examination/consultation
The examination before inserting the Mirena system may include a cervical smear as well as other tests, such as infection screening, including sexually transmitted diseases if necessary. A gynecological examination should be performed to determine the position and size of the uterus.
Mirena is not a suitable contraceptive method for use immediately after unprotected sexual intercourse (postcoital, emergency contraception).
Infections
The applicator tube helps protect the Mirena system from microbial contamination during insertion. The Mirena applicator has been designed to reduce the risk of infection. Nevertheless, there is an increased risk of infection shortly after insertion and during the first month after insertion of the Mirena system. Pelvic inflammatory disease in women using the Mirena system is often associated with sexually transmitted diseases. The risk of infection increases with multiple sexual partners. Pelvic inflammatory disease must be properly treated, as it may affect fertility and increase the risk of ectopic pregnancy. The Mirena system should be removed in case of recurring endometritis or pelvic inflammatory disease, if there are acute and severe inflammatory conditions or if they do not resolve after a few days of treatment.
In extremely rare cases, shortly after insertion of the intrauterine therapeutic system, severe infection or sepsis (a very severe infection that can be life-threatening) may occur.
You should contact your doctor immediately if you experience persistent abdominal pain, elevated body temperature, pain during sexual intercourse, or abnormal vaginal bleeding.
Expulsion
Uterine contractions during menstrual bleeding may sometimes cause the system to move or be expelled. This is more likely if the woman is overweight at the time of insertion or if she has had heavy menstrual bleeding in the past. If the system is not in its proper position, it may not work as intended, and the risk of pregnancy increases. Expulsion of the Mirena system results in loss of pregnancy protection.
Possible symptoms of expulsion include vaginal bleeding or abdominal pain, but Mirena may also be expelled unnoticed. Since Mirena reduces menstrual bleeding, the intensity of these bleedings may be a sign of expulsion or system displacement.
It is recommended to check with your fingers (e.g., during bathing) whether the threads are in the correct position. See also section 3 "How to use Mirena - Self-examination of the correct position of the Mirena system". If symptoms occur that may indicate expulsion of the system or if the threads cannot be felt in the cervical area, you should use other contraceptive methods (such as condoms) and contact your doctor.
Perforation of the uterus
Perforation or damage to the uterine wall may occur, most often during insertion, although it may only be detected later. A Mirena system that is outside the uterine cavity is not effective in preventing pregnancy and should be removed as soon as possible. Removal of the system may require surgery. The risk of perforation is higher in breastfeeding women and within 36 weeks after delivery; this risk may also be increased in women with a permanently retroverted uterus (uterus tilted backwards). If you suspect perforation of the uterus, you should consult your doctor and inform them that you have a Mirena system, especially if it is not the doctor who inserted the system.
Possible signs and symptoms of perforation may include:
- severe pain (like menstrual cramps) or more severe pain than expected
- heavy bleeding (after insertion)
- pain or bleeding that lasts longer than a few weeks
- sudden changes in your periods
- pain during sexual intercourse
- inability to feel the threads of the Mirena system (see section 3 "How to use Mirena - Self-examination of the correct position of the Mirena system").
Ectopic pregnancy
Getting pregnant while using the Mirena system is very unlikely. However, if you get pregnant while using the Mirena system, the likelihood of an ectopic pregnancy is relatively increased. In about 1 in 1000 women who properly used the Mirena system, an ectopic pregnancy occurred within a year of use. This is less than in women who do not use any contraceptive methods (about 3 to 5 in 1000 women per year). In women who have had an ectopic pregnancy in the past, have had tubal surgery, or have had pelvic inflammatory disease, there is an increased risk of ectopic pregnancy.
Ectopic pregnancy is a serious condition that requires immediate medical attention.
Symptoms that may indicate an ectopic pregnancy and require immediate medical attention include:
- cessation of menstrual bleeding, followed by persistent bleeding or pain
- dull or very severe pain in the lower abdomen
- typical symptoms of pregnancy with concurrent bleeding and dizziness.
Weakness
Some women may experience dizziness after insertion of the system. This is a normal physiological reaction.
Your doctor will recommend resting after insertion of the Mirena system.
Ovarian cysts enclosing a mature ovum in the ovary
The contraceptive properties of the Mirena system are related to its local action, so in women of childbearing age, menstrual cycles are usually ovulatory and ovulation occurs. Sometimes, an unruptured follicle does not regress for a while and may enlarge. In most cases, these enlarged follicles do not cause any symptoms, although they may cause pelvic pain or pain during sexual intercourse. Such enlarged ovarian cysts usually resolve on their own, but may also require medical intervention.
Mirena and other medicines
Since the mechanism of action of the Mirena system is primarily local, taking other medicines should not increase the risk of pregnancy during use of this system.
However, it is recommended that you tell your doctor about all medicines you have taken recently, including those that are available without a prescription.
Pregnancy, breastfeeding, and fertility
Pregnancy
Mirena should not be used during pregnancy or if pregnancy is suspected.
It is very rare for a woman to become pregnant when the Mirena system is in place. However, if the Mirena system is displaced, contraceptive protection is reduced, and other contraceptive methods should be used until a doctor's visit.
While using the Mirena system, some women may experience cessation of menstrual bleeding after a while. Lack of menstrual bleeding does not always mean pregnancy. If menstrual bleeding has stopped and other signs of pregnancy are present (e.g., nausea, fatigue, breast tenderness), you should consult a doctor for an examination and a pregnancy test.
If you become pregnant while using the Mirena system, you should contact your doctor immediately to have the system removed. Removal may cause miscarriage. However, leaving the Mirena system in place during pregnancy may increase not only the risk of miscarriage but also the risk of preterm birth. If the Mirena system cannot be removed, you should discuss the benefits and risks of continuing the pregnancy with your doctor. If the pregnancy is continued, it should be monitored closely by a doctor, and you should immediately report any symptoms such as abdominal cramps, abdominal pain, or fever to your doctor.
Mirena contains a hormone called levonorgestrel, and there have been single reports of its effect on the genital organs of girls exposed to levonorgestrel released from an intrauterine device remaining in the uterine cavity.
Breastfeeding
Mirena can be used during breastfeeding. Levonorgestrel passes into breast milk in small amounts (about 0.1% of the levonorgestrel dose may pass into the infant's body through breast milk). After 6 weeks postpartum, use of the Mirena system has no adverse effect on the growth and development of the infant. It has not been shown that progestogen-only contraceptives affect the amount and quality of milk.
Hormonal contraception is not recommended as a first-line method during breastfeeding; only non-hormonal methods are recommended. Progestogen-only contraceptive methods, such as the Mirena system, are second-line methods. The daily dose and blood levels of levonorgestrel are lower than with other hormonal contraceptive methods.
Fertility
Removal of the Mirena system restores normal fertility in women.
If you are pregnant or breastfeeding, or suspect that you are pregnant or plan to become pregnant, you should consult a doctor or pharmacist before taking this medicine.
Driving and using machines
No effects of the Mirena system on the ability to drive and use machines have been observed.
Important information about some components of the Mirena system
The T-shaped frame of the Mirena system contains barium sulfate, which allows the system to be visualized during X-ray examination.
3. How to use Mirena
Efficacy of the Mirena system
The contraceptive efficacy of the Mirena system is the same as that of the most effective copper-containing intrauterine device. Clinical trials have shown about 2 pregnancies per 1000 women using the Mirena system in the first year.
In the treatment of excessive menstrual bleeding of unknown origin, the intensity of bleeding decreases after 3 months of using the Mirena system. In some women, menstrual bleeding may even stop.
Insertion of the Mirena system
The Mirena system is inserted within 7 days of the onset of menstrual bleeding. The system can also be inserted immediately after a miscarriage in the first trimester, provided there is no infection of the genital tract. The system can only be inserted when the uterus has returned to its normal size after delivery and not earlier than 6 weeks after delivery (see section 2 - "Important information before using Mirena", subsection "Perforation of the uterus"). The Mirena system can be replaced with a new one at any time during the cycle.
The Mirena system should only be inserted by a doctor or healthcare professional with experience in inserting the system.
Method of inserting the Mirena system
After a gynecological examination, a speculum is inserted into the vagina and the cervix is rinsed with an antiseptic solution. The intrauterine system is then inserted into the uterus using a thin, flexible plastic tube (applicator). If necessary, the cervix can be locally anesthetized before insertion.
Some people may experience pain and dizziness after insertion of the system. If these do not resolve within half an hour while the patient is in a lying position, it may indicate that the system has been inserted incorrectly. An examination should be performed and the system removed if necessary.
After insertion of the Mirena system, the patient should receive a reminder card from the doctor, which should be brought to each scheduled visit.
When to consult a doctor
The doctor should check the presence of the system within 4 to 12 weeks after its insertion and then regularly check the presence of the system at least once a year. The doctor will determine individually how often and what control examinations should be performed. The patient should bring the reminder card received from the doctor to each scheduled visit.
In addition, you should consult a doctor if:
- the threads are not felt in the vagina
- the lower part of the system is felt
- you suspect you are pregnant
- you experience persistent abdominal pain, fever, or abnormal vaginal discharge
- you or your partner experience pain or discomfort during sexual intercourse
- you experience sudden changes in your menstrual cycle (e.g., scant or absent menstrual bleeding, followed by persistent bleeding or pain)
- you experience other health problems, such as migraines or severe headaches, sudden vision problems, jaundice, or increased blood pressure
- any of the conditions listed in section 2 "Important information before using Mirena" occur.
Remind your doctor that you have a Mirena system, especially if it is not the doctor who inserted the system.
Duration of use of the Mirena system
The Mirena system prevents pregnancy (has a contraceptive effect) for 8 years after insertion. If the patient uses the Mirena system for this purpose, the system should be removed or replaced no later than 8 years after insertion.
The Mirena system is effective for 5 years after insertion in the treatment of excessive menstrual bleeding (of unknown origin). If the patient uses Mirena for this purpose, the system should be removed or replaced when excessive menstrual bleeding returns or no later than 8 years after insertion. If the patient wishes, a new system can be inserted after removal of the previous one.
If you want to remove the Mirena system to become pregnant or for other reasons
Your doctor can easily remove the system at any time, and then it is possible to become pregnant.
Removal of the system is usually painless. After removal of the Mirena system, fertility returns.
If you do not plan to become pregnant, the Mirena system should not be removed after the 7th day of the menstrual cycle (unless other contraceptive methods are used, such as condoms, for at least 7 days before removal). If you have irregular menstrual periods or do not have menstrual periods, you should use mechanical contraceptive methods for at least 7 days before removal and until menstrual bleeding resumes. You can also have a new system inserted immediately after removal of the previous one, and in this case, no additional protection is required.
Can you become pregnant after stopping the use of the Mirena system
Yes. Removal of the Mirena system does not disrupt fertility. You can become pregnant during the first menstrual cycle after removal of the Mirena system.
Does the Mirena system affect menstrual bleeding
The Mirena system does not affect the menstrual cycle. The system may cause various changes in menstrual bleeding, such as spotting (minor blood loss), shorter or longer bleeding, scant or heavy bleeding, or its absence.
In many women, during the first 3 to 6 months after insertion of the Mirena system, frequent spotting or minor bleeding occurs outside of menstrual bleeding. In some women, menstrual bleeding may become heavier or longer than usual. You should inform your doctor, especially if these symptoms do not resolve.
Generally, it is possible to gradually reduce the number of days of bleeding and the amount of blood lost each month.
In some women, menstrual bleeding may eventually stop completely. As the Mirena system usually reduces menstrual bleeding, many women experience an increase in hemoglobin levels in the blood.
After removal of the system, menstrual periods return to normal.
Is the absence of menstrual bleeding a normal condition
Yes, when using the Mirena system. The absence of menstrual periods is a sign of the effect of the hormone on the uterine lining. There are no monthly changes in the uterine lining. Therefore, there is nothing that would normally be expelled with menstrual blood. This does not have to be a sign of menopause or pregnancy. Hormone levels remain normal.
In fact, the absence of menstrual periods can be a great benefit for a woman's health.
Pregnancy diagnosis
Becoming pregnant while using the Mirena system is unlikely, even if menstrual bleeding does not occur.
If menstrual bleeding has not occurred for 6 weeks and this causes concern, a pregnancy test can be performed.
If the result is negative, there is no need for further testing, unless other symptoms of pregnancy are present, such as nausea, fatigue, or breast tenderness.
Can the Mirena system cause pain or discomfort
Some women experience pain (like menstrual cramps) for a few weeks after insertion of the system. You should consult your doctor or clinic again if you experience severe pain or if pain persists for more than 3 weeks after insertion of the Mirena system.
Effect of the Mirena system on sexual intercourse
Neither the patient nor her partner should feel the system during sexual intercourse. However, if they do, you should avoid sexual intercourse until the doctor checks whether the system is still in the correct position.
How soon after insertion of the system can sexual intercourse occur
To allow the body to rest, it is recommended to wait about 24 hours after insertion of the system before having sexual intercourse. Nevertheless, the Mirena system prevents pregnancy immediately after insertion.
Using tampons or menstrual cups
It is recommended to use sanitary pads. If you use tampons or menstrual cups, you should change them carefully to avoid pulling on the threads of the Mirena system. If you think the Mirena system has been displaced from its correct position (see "When to consult a doctor" with possible symptoms), you should avoid sexual intercourse or use mechanical contraception (such as condoms) and consult your doctor.
What happens if the Mirena system is expelled
Rarely, but it is possible, that the Mirena system may be expelled without the patient's knowledge during menstrual bleeding. If menstrual bleeding is heavier than usual, it may indicate that the Mirena system has been expelled through the vagina. It is also possible for the Mirena system to be partially expelled from the uterus (the patient or partner may notice this during sexual intercourse). If the Mirena system is completely or partially expelled, it does not protect against pregnancy.
Self-examination of the correct position of the Mirena system
A woman can check herself whether the threads of the system are in the correct position. To do this, she should carefully insert her finger into the vagina and check for the presence of the threads near the cervix.
Do not pull on the threads, as this can cause the system to be accidentally removed. If the threads are not felt, it may indicate that the system has been expelled from the uterus or that the uterus has been perforated. In this case, you should use mechanical contraception (such as condoms) and consult your doctor.
4. Possible side effects
Like all medicines, Mirena can cause side effects, although not everybody gets them.
In addition to the possible side effects listed in other sections (e.g., section 2 "Important information before using Mirena"), the following possible side effects are listed below, divided by body system and frequency of occurrence.
Very common:may occur in 1 in 10 patients
Reproductive system and breast disorders
- uterine or vaginal bleeding, including spotting, infrequent periods, or their absence
- mild ovarian cysts (see section 2 "Ovarian cysts")
Common:may occur in 1 to 10 in 100 patients
Psychiatric disorders
- depressed mood or depression
- nervousness
- decreased libido
Nervous system disorders
- headache
Vascular disorders
- dizziness
Gastrointestinal disorders
- abdominal pain
- nausea
Skin and subcutaneous tissue disorders
- acne
Musculoskeletal and connective tissue disorders
- back pain
Reproductive system and breast disorders
- pelvic pain
- painful menstruation
- discharge
- vulvovaginitis
- breast tenderness
- breast pain
- expulsion of the intrauterine therapeutic system
Diagnostic tests
- weight gain
Uncommon:may occur in 1 to 10 in 1000 patients
Nervous system disorders
- migraine
Gastrointestinal disorders
- abdominal bloating
Skin and subcutaneous tissue disorders
- hirsutism (male-type hair growth in women)
- hair loss (alopecia)
- pruritus (severe itching)
- rash (skin inflammation)
- chloasma (brown spots on the skin) or intense skin discoloration
Reproductive system and breast disorders
- perforation (puncture) of the uterus
- pelvic inflammatory disease (infection of the upper female reproductive tract, organs above the cervix)
- endometritis
- cervicitis - normal class II Papanicolau smear in cytological examination (cervicitis)
General disorders and administration site conditions
- edema
Rare:may occur in 1 to 10 in 10,000 patients
Skin and subcutaneous tissue disorders
- urticaria
- rash
If you become pregnant while using Mirena, there is a likelihood that the pregnancy will be ectopic (see section 2 "Ectopic pregnancy").
After insertion of the intrauterine therapeutic system, cases of sepsis (a very severe infection that can be life-threatening) have been reported.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Mirena
The medicine should be stored out of sight and reach of children.
There are no special precautions for storage. Store in the original packaging.
Do not insert Mirena after the expiry date stated on the packaging. The expiry date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer used. This will help protect the environment.
6. Contents of the packaging and other information
What Mirena contains
- The active substance of Mirena is levonorgestrel. One intrauterine therapeutic system contains 52 mg of levonorgestrel.
- The other ingredients of Mirena are: polydimethylsiloxane elastomer, polydimethylsiloxane tubing - contains anhydrous colloidal silica (30-40%), T-body composition:polyethylene containing 20-24% barium sulfate, threads composition:polyethylene, black iron oxide (E 172).
What Mirena looks like and contents of the packaging
Package size: one sterile packaged intrauterine therapeutic system for intrauterine use.
For more detailed information, you should consult a doctor, the marketing authorization holder, or the parallel importer.
Marketing authorization holder in Romania, the country of export:
Bayer AG, Kaiser-Wilhelm-Allee 1, 51373 Leverkusen, Germany
Manufacturer:
Bayer Oy, Pansiontie 47, 20210 Turku, Finland
Parallel importer:
InPharm Sp. z o.o., ul. Strumykowa 28/11, 03-138 Warsaw
Repackaged by:
InPharm Sp. z o.o. Services sp. k., ul. Chełmżyńska 249, 04-458 Warsaw
Romanian marketing authorization number, country of export:7842/2015/01
Parallel import authorization number:379/22
Date of approval of the leaflet: 04.07.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Bayer AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MirenaDosage form: System, 19.5 mg/systemActive substance: plastic IUD with progestogenManufacturer: BAYER OyPrescription requiredDosage form: System, 52 mg (20 mcg/24h)Active substance: plastic IUD with progestogenPrescription requiredDosage form: System, 52 mg (20 mcg/24 h)Active substance: plastic IUD with progestogenPrescription required
Alternatives to Mirena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mirena in Ukraine
Alternative to Mirena in Spain
Online doctors for Mirena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mirena – subject to medical assessment and local rules.