LEVOSERT ONE 0.02mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM


How to use LEVOSERT ONE 0.02mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the user
Levosert One0.02 mg every 24 hours intrauterine release system
levonorgestrel
Read the entire leaflet carefully before starting to use this medication,as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Levosert One and what is it used for
- What you need to know before starting to use Levosert One
- How to use Levosert One
- Possible side effects
5 Preservation of Levosert One
- Package contents and additional information
1. What is Levosert One and what is it used for
This medication is an intrauterine release system (IUS) for insertion into the uterus, where it slowly releases the hormone levonorgestrel.
It is used for:
Contraception
This medication is an effective, long-term, non-permanent (reversible) contraceptive method.
This medication prevents pregnancy by thinning the lining of the uterus (endometrium), making the normal mucus of the uterine opening (cervical canal) thicker, so that sperm cannot pass through to fertilize the egg, and preventing the release of eggs (ovulation) in some women. Additionally, the presence of the T-shaped body causes local effects on the uterine lining.
The system should be removed after 8 years of use when used as a contraceptive.
Treatment of heavy menstrual bleeding
This medication is also useful for reducing menstrual blood flow, so you can use it if you suffer from heavy menstrual bleeding (menorrhagia). The hormone in this medication acts by thinning the lining of your uterus so that there is less bleeding each month.
The system should be removed or replaced after 8 years of use, or earlier if heavy or bothersome menstrual bleeding returns.
Children and adolescents
This medication is not indicated for use before the first menstrual period (menarche).
2. What you need to know before starting to use Levosert One
Do not use Levosert One
- if you are pregnant or think you may be pregnant;
- if you have or have had pelvic inflammatory disease;
- if you have an unusual or unpleasant vaginal discharge, or vaginal itching, as this may indicate an infection;
- if you have or have had inflammation of the uterine lining after childbirth;
- if you have or have had an infection in the uterus after childbirth or after an abortion in the last 3 months;
- if you have or have had inflammation of the cervix (the neck of the uterus);
- if you have or have had an abnormal Pap test (changes in the cervix);
- if you have or have had liver problems;
- if you have a liver tumor;
- if you have a uterine anomaly, including uterine fibroids, especially those that distort the uterine cavity;
- if you have an abnormal vaginal bleeding pattern;
- if you have a condition that makes you susceptible to infections. A doctor will have told you if you have this type of condition;
- if you have or have had hormone-dependent cancer, such as breast cancer;
- if you have or have had or suspect the existence of any type of cancer, including blood cancer (leukemia), uterine, and cervical cancer, unless you are in remission;
- if you have or have had trophoblastic disease. A doctor will have told you if you have this type of disease;
- if you are allergic to levonorgestrel or any of the other components of this medication (listed in section 6).
Warnings and precautions
Before this medication is inserted, your doctor or nurse will perform some tests to ensure that this medication is suitable for you. This will include a pelvic exam and may also include other tests, such as a breast exam, if your doctor or nurse considers it appropriate.
Genital infections will need to be successfully treated before this medication can be inserted.
If you have epilepsy, inform your doctor or nurse before inserting this medication, as it can rarely cause a seizure during insertion. Some women may feel faint after the procedure. This is normal, and your doctor or nurse will tell you to rest for a while.
This medication may not be suitable for all women.
This medication, like other hormonal contraceptives, does not protect against HIV (AIDS) or any other sexually transmitted disease (e.g., chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis). You will need to use condoms to protect yourself from these diseases.
Talk to your doctor before using Levosert One:
- if you have or develop migraines, dizziness, blurred vision, worse-than-usual headaches, or if you have headaches more frequently than before;
- if you have a yellow color in the skin or whites of the eyes (jaundice);
- if you are diabetic (high blood sugar level), have high blood pressure, or abnormal blood lipid levels;
- if you have had blood cancer (including leukemia) that is now in remission
- if you are undergoing long-term steroid treatment;
- if you have had an ectopic pregnancy (fetal development outside the uterus) or a history of ovarian cysts;
- if you have had a severe arterial disease, such as a heart attack or stroke;
- if you have a history of blood clots (thrombosis);
- if you are taking other medications, as certain medications can prevent this medication from working properly;
- if you have irregular bleeding;
- if you have seizures (epilepsy).
If you have or have had any of the above conditions, your doctor will decide if you can use this medication.
You should also inform your doctor if any of these conditions occur for the first time while you have this medication inserted.
You should see a doctor or nurse as soon as possible if you experience painful swelling in the leg, sudden chest pain, or difficulty breathing, as these can be signs of a blood clot. It is essential that any blood clot is treated immediately.
Expulsion
Uterine muscle contractions during menstruation can sometimes push the IUS out of place or expel it. This is more likely to happen if you are overweight at the time of IUS insertion or if you have a history of heavy menstrual periods. If the IUS is displaced, it may not work properly, and the risk of pregnancy increases. If the IUS is expelled, you are no longer protected against pregnancy.
Possible symptoms of expulsion are pain and abnormal bleeding, but Levosert One can also be expelled without you realizing it. Because Levosert One reduces menstrual flow, an increase in flow may indicate an expulsion.
It is recommended that you check the threads with your finger, for example, while showering. See also section 3 "How to use Levosert One - How can I tell if Levosert One is in place?". If you experience signs that indicate expulsion or are unable to feel the threads, you should use an additional contraceptive method (such as condoms) and consult your healthcare professional.
Psychiatric disorders:
Some women using hormonal contraceptives like this medication have reported depression or a depressed mood. Depression can be severe and sometimes can lead to suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor for additional medical advice as soon as possible.
This medication and smoking
Women are advised to stop smoking. Smoking increases the risk of developing a heart attack, stroke, or blood clots.
Use of tampons and menstrual cups
The use of pads is recommended. If tampons or menstrual cups are used, they should be changed carefully to avoid pulling on the removal threads of Levosert One.
Other medications and Levosert One
The effect of hormonal contraceptives like this medication can be reduced by medications that increase the amount of liver enzymes produced. Inform your doctor if you are taking:
- phenobarbital, phenytoin, or carbamazepine (for epilepsy);
- griseofulvin (an antifungal);
- rifampicin or rifabutin (antibiotics);
- nevirapine or efavirenz (for HIV).
Inform your doctor if you are taking, have recently taken, or may need to take any other medication. This medication should not be used at the same time as another hormonal contraceptive.
Pregnancy, breastfeeding, and fertility
Do not use this medication during pregnancy or if you suspect you may be pregnant.
Can I get pregnant while using this medication?
It is very rare for a woman to become pregnant while using this medication.
Not having a period does not necessarily mean you are pregnant. Some women may not have periods while using the system.
If you have not had a period for 6 weeks, consider taking a pregnancy test. If it is negative, there is no need for further testing, unless you have other symptoms of pregnancy, such as nausea, fatigue, or breast tenderness.
If you become pregnant with the device in place, contact your doctor as soon as possible to rule out an ectopic pregnancy (fetal development outside the uterus) and to have this medication removed to reduce the risk of a spontaneous abortion. However, if Levosert One is left in place during pregnancy, it not only increases the risk of spontaneous abortion but also the risk of preterm birth. If Levosert One cannot be removed, talk to your healthcare professional about the benefits and risks of continuing the pregnancy. If the pregnancy continues, you will be closely monitored during your pregnancy, and you should contact your doctor immediately if you experience stomach cramps, stomach pain, or fever.
Levosert One contains a hormone called levonorgestrel, and there have been isolated cases of genital effects in babies if they are exposed to levonorgestrel intrauterine devices while in the uterus.
What if I want to have a baby?
If you want to have a baby, ask your doctor to remove this medication. Your normal level of fertility will return very quickly once the system is removed.
Can I breastfeed while using this medication?
Very small amounts of the hormone from this medication are found in breast milk. No risk to the newborn is expected. You can continue breastfeeding during the use of this medication.
Driving and using machines
There are no known effects on the ability to drive and use machines.
Levosert One contains barium sulfate
The T-shaped structure of this medication contains barium sulfate, which makes it visible on X-rays.
3. How to use Levosert One
Only a doctor or nurse with specific training can insert the system (see special instructions for insertion in the package).
The professional will explain the placement procedure and any risks associated with its use. Afterwards, you will be examined by your doctor or nurse before the insertion of this medication. If you have any doubts about its use, you can consult with them.
Starting to use Levosert One
- Before inserting Levosert One, it is necessary to ensure that you are not pregnant.
- You should have Levosert One inserted within 7 days of the start of your menstruation. When Levosert One is inserted during these days, it acts immediately and will prevent you from becoming pregnant.
- If you cannot have Levosert One inserted within 7 days of the start of your menstruation or if your menstrual period arrives at unpredictable times, Levosert One can be inserted on any other day. In this case, you should not have had unprotected sex since your last menstrual period, and you should have a negative pregnancy test before insertion. Additionally, Levosert One may not prevent pregnancy reliably from the start. Therefore, you should use a barrier contraceptive method (such as condoms) or abstain from vaginal sex for the first 7 days after the insertion of Levosert One.
- Levosert One is not suitable for use as an emergency contraceptive (postcoital contraceptive).
Starting to use Levosert One after childbirth
- Levosert One can be inserted after childbirth once the uterus has returned to its normal size, but not before 6 weeks after childbirth (see section 4 "Possible side effects - Perforation").
- Also, see "Starting to use Levosert One" above to learn more about what you need to know about the time of insertion.
Starting to use Levosert One after an abortion
Levosert One can be inserted immediately after an abortion, if the pregnancy was less than 3 months long and there are no genital infections. Then, Levosert One will work immediately.
Replacing Levosert One
Levosert One can be replaced with a new Levosert One at any time during your menstrual cycle. Then, Levosert One will work immediately.
Switching from another contraceptive method (such as combined hormonal contraceptives, implant)
- Levosert One can be inserted immediately if there is reasonable certainty that you are not pregnant.
- If it has been more than 7 days since the start of your menstrual bleeding, you should abstain from vaginal sex or use additional contraceptive protection for the next 7 days.
Insertion of Levosert One
The examination performed by your healthcare professional before insertion may include:
- a cervical cytology test (Pap smear);
- a breast examination;
- other tests, for example for infections, including sexually transmitted diseases, pregnancy test, as necessary. Your healthcare professional will also perform a gynecological examination to determine the position and size of the uterus.
After the gynecological examination
- An instrument called a speculum is inserted into the vagina, and the cervix may be cleaned with an antiseptic solution. Then, Levosert One is placed in the uterus using a thin, flexible plastic tube (the insertion tube). Local anesthesia may be applied to the cervix before insertion.
- Some women feel dizzy or faint during or after the insertion or removal of Levosert One.
- You may experience some pain and bleeding during or immediately after insertion.
After the insertion of Levosert One, you should receive a patient reminder card from your doctor for follow-up appointments. Bring this card with you to each scheduled appointment.
How quickly does Levosert One work?
Contraception
If Levosert is inserted into your uterus during your menstrual period or within 7 days after the start of your period, or if you have a device and it's time to replace it with a new one, or if you have just had an abortion, you are protected against pregnancy from the moment the system is inserted.
Heavy menstrual bleeding
This medication normally achieves a significant reduction in menstrual blood loss within 3 to 6 months of treatment.
How will Levosert One affect my periods?
Many women experience spotting (a small amount of blood loss) in the first 3-6 months after the insertion of the system. Others will have prolonged or heavy bleeding. However, you may experience an increase in bleeding, usually in the first 2 to 3 months, before a reduction in blood loss is achieved. In general, you are more likely to have fewer days of bleeding each month and may even stop having your period. This is due to the effect of the hormone (levonorgestrel) on the uterine lining. If a notable reduction in blood loss is not achieved within 3 to 6 months, other treatments should be considered.
If you have had this medication inserted for a long time and then start experiencing bleeding problems, contact your doctor or healthcare professional for advice.
How often should I have my system checked?
You should check your Levosert One 4 to 6 weeks after insertion, and then regularly, at least once a year until its removal. Your doctor will determine how often and what types of checks are required in your particular case. Bring the patient reminder card you received from your doctor to each scheduled appointment. Also, you should contact your doctor if you experience any of the symptoms described in section 2 "Warnings and precautions".
How can I know if the system is in place?
After each menstrual period, you can look for the two thin threads that are attached to the lower end of the system. Your doctor will show you how to do this.
Do not pullon the threads, as you could accidentally pull it out. If you cannot find the threads, contact your doctor or nurse as soon as possible and avoid sex or use a barrier contraceptive method (such as condoms) in the meantime. The threads may have simply entered the uterus or cervical canal. If your doctor or nurse still cannot find the threads, they may have broken, or the medication may have come out on its own, or in rare cases, it may have perforated the uterine wall (uterine perforation, see section 4).
You should also go to the doctor if you can touch the lower end of the device itself, or if you or your partner experience pain or discomfort during sex.
If the system comes out completely or partially, you may not be protected against pregnancy. It is rare, but possible, that this happens without you realizing it during your menstrual period. An unusual increase in bleeding during your period may be a sign that this has happened. Inform your doctor or healthcare professional if you experience unexpected changes in your bleeding pattern.
Removal of Levosert One
Levosert One should be removed or replaced after 8 years of use, or earlier if heavy or bothersome menstrual bleeding returns.
Your doctor can easily remove the system at any time, after which it is possible to become pregnant. Some women feel dizzy or faint during or after the removal of Levosert One. You may experience some pain and bleeding during the removal of Levosert One.
Continuation of contraception after removal
If you do not want to become pregnant, Levosert One should not be removed after the seventh day of the menstrual cycle (menstrual period) unless you use other contraceptive methods (e.g., condoms) for at least 7 days before the removal of the IUD.
If you have irregular periods or do not have periods, you should use a barrier contraceptive method for 7 days before removal.
Also, a new Levosert One can be inserted immediately after removal, in which case no additional protection is needed. If you do not want to continue with the same method, ask your doctor about other reliable contraceptive methods.
If you have more questions about the use of this medication, ask your doctor.
4. Possible side effects
Like all medications, this medication can cause side effects, although not all people experience them.
With this medication, side effects are more frequent during the first months after the system has been inserted and decrease over time.
If you experience any of the following serious side effects, please contact your doctor or nurse immediately:
- Severe pain or fever that develops shortly after insertionmay indicate that you have a serious infection that should be treated immediately. In rare cases, a very serious infection (sepsis) may occur.
- Severe pain and continued bleedingas this may be a sign of damage or tearing of the uterine wall (perforation). Perforation is rare, but it occurs more frequently during the insertion of this medication, although it may not be detected until some time later. If this medication has become lodged outside the uterine cavity, it is not effective in preventing pregnancy and should be removed as soon as possible; in very rare cases, this may require surgery. The risk of perforation is low, but it increases in breastfeeding women or women who have had a baby up to 36 weeks before insertion and may increase in women with a fixed retroverted uterus. If you suspect that you may have suffered a perforation, seek medical attention immediately and remind them that you have this medication inserted, especially if it was not the person who inserted it.
Possible signs and symptoms of perforation may include:
- severe pain (like menstrual cramps) or more pain than expected
- heavy bleeding (after insertion)
- pain or bleeding that continues for more than a few weeks
- sudden changes in periods
- pain during sex
- if you can no longer feel the threads of this medication (see section 3 "How to use Levosert "How can I know if the system is in place?").
- Lower abdominal pain, especially if you also have a fever or have had a missed or unexpected bleeding, as this may be a sign of ectopic pregnancy (fetal development outside the uterus). The absolute risk of ectopic pregnancy in users of this medication is low. However, when a woman becomes pregnant with this medication in place, the likelihood of ectopic pregnancy increases.
- Lower abdominal pain or experiencing difficult or painful sexas this may be a sign of ovarian cysts or pelvic inflammatory disease. This is important because pelvic infections can reduce your chances of having a baby and can increase the risk of ectopic pregnancy.
Other side effects
Very common(may affect more than 1 in 10 women) may include:
- absence of menstruation, light or infrequent (see "How will Levosert One affect my menstruation?" in section 3.
- vaginal bleeding, including spotting.
- vaginal and external genital infections (vulva) caused by fungi or bacteria;
- acne;
Common(may affect up to 1 in 10 women) may include:
- depression, nervousness, or other mood changes;
- reduced sexual desire;
- headache;
- migraine;
- feeling of fainting (presyncope);
- dizziness;
- back pain;
- abdominal discomfort;
- nausea;
- bloating;
- vomiting;
- painful periods;
- increased vaginal discharge;
- sensitive and painful breasts;
- uterine spasms;
- This medication comes out of place;
- weight gain.
Uncommon(may affect up to 1 in 100 women) may include:
- fainting;
- eczema;
- cervicitis (inflammation of the cervix);
- swelling or inflammation in the legs or ankles;
- increased hair growth on the face and body;
- hair loss;
- itching of the skin (pruritus);
- skin discoloration or increased skin pigmentation, especially on the face (chloasma).
Rare(may affect up to 1 in 1,000 women) may include:
- skin rash, itching.
Reporting side effects
If you experience side effects, consult your doctor or pharmacist, even if they are possible side effects that do not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Levosert One
This medication does not require special storage conditions.
Keep in the original packaging and keep the blister sealed in the outer box to protect it from light.
Keep the container perfectly closed. Only your doctor or healthcare professional should open it.
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the label and on the box after "EXP". The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This way, you will help protect the environment.
6. Container Content and Additional Information
Composition of Levosert One
The active ingredient is levonorgestrel.
Levosert One contains 52 mg of levonorgestrel contained in a substance called polydimethylsiloxane, surrounded by a membrane also of polydimethylsiloxane.
Appearance of the Product and Container Content
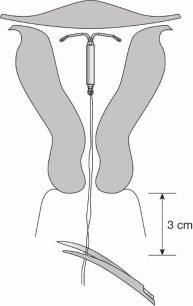
- Levosert One is formed by a T-shaped body made of a plastic called polyethylene. This structure carries a hormone reservoir that allows it to be released gradually into the uterus.
- There are two fine threads, made of polypropylene and copper phthalocyanine blue, attached to the lower end of this structure. These threads will allow for easy removal of the device and for you and your doctor to check that the device is in place.
The Levosert One SLI along with the applicator device is presented individually packaged in a thermoformed plastic blister pack with a removable cover inside a cardboard box. The sterile blister is packaged inside a box with the prospectus and the patient reminder card.
Package sizes:
1 intrauterine release system with applicator device.
Multipack with 5 packages with an intrauterine release system with an applicator device.
Only some package sizes may be marketed.
Marketing Authorization Holder
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungary
Manufacturer
Odyssea Pharma S.A.
Rue du Travail 16
4460 Grâce Hollogne
Belgium
Gedeon Richter Plc.
Gyömroi út 19-21
1103 Budapest
Hungary
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Gedeon Richter Ibérica S.A.
Sabino Arana, 28 4º 2ª
08028 Barcelona
Spain
+34 93 2034300
This medication is authorized in the member states of the European Economic Area under the following names:
Austria Levosert One
Cyprus Levosert One
Germany Levosert One
Denmark Levosert One
Spain Levosert One 0.02 mg every 24 hours intrauterine release system
Ireland Levosert SHI
Iceland Levosertone
Italy Benilexa
Malta Levosert One
Norway Levosert Single-Handed Inserter
Sweden Levosert Single-Handed Inserter
Slovenia Levosert SHI
United Kingdom Benilexa One Handed
Date of the last revision of thisprospectusJune 2024
Other sources of information
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
_________________________________________________________________________________
This information is intended only for healthcare professionals:
Instructions for use and handling
Checklist for the prescribing doctor
Ask yourself the following questions before prescribing/inserting this medication:
- Have I checked that the patient's needs meet the indications for contraception or heavy menstrual bleeding and the duration of use, up to eight years?
- Have I completed the patient's card included in the package and given it to the patient as a reminder? (Any insertion of more than eight years of duration must be reported as unauthorized use)
Read the following instructions for use carefully, as there may be some differences in the type of applicator device compared to other IUDs you have used before:
Insertion instructions
It must be inserted by a healthcare professional using an aseptic technique.
It is recommended that Levosert One should only be inserted by healthcare professionals who are experienced in the placement of intrauterine release systems (IUS) and/or who have received sufficient training on the insertion procedure of Levosert One and have read these instructions carefully before inserting Levosert One.
Levosert One is supplied in a sterile package that should not be opened until it is necessary for its insertion. Do not re-sterilize. For single use. The product, once exposed, must be handled with aseptic precautions. If the seal of the sterile package is broken, the product must be discarded (see disposal instructions in section 6.6). Do not use if the inner package is damaged or open. Do not insert after the expiration date indicated on the box and on the blister after CAD. The expiration date is the last day of the month indicated.
To know the time of insertion, consult section 4.2 of the technical data sheet.
Levosert One includes a patient reminder card in the package. Complete the patient reminder card and give it to the patient after insertion.
Preparation for insertion
- Examine the patient to rule out contraindications for the insertion of Levosert One (see sections 4.3 and 4.4 under Medical Examination).
- Place a speculum, visualize the cervix, and then perform a thorough cleaning of the cervix and vagina with a suitable antiseptic solution.
- The healthcare professional may have the collaboration of auxiliary personnel if necessary.
- Hold the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is in retroversion, it may be more appropriate to hold the posterior lip of the cervix. A gentle traction can be applied with the forceps to straighten the cervical canal. The forceps must remain in place and gentle traction must be maintained on the cervix throughout the insertion procedure.
- Insert a uterine sound through the cervical canal to the fundus to measure the depth. If the uterine depth is <5.5 cm, interrupt the procedure. Confirm the direction of the uterine cavity and rule out any evidence of intrauterine anomalies (e.g., septum, submucous myomas) or a previously inserted intrauterine contraceptive that has not been removed. If difficulty is encountered, consider cervical dilation. If cervical dilation is required, consider the use of analgesics and/or a paracervical block.
Description
Figure 1a: Intrauterine Release System (IUS) Levosert One

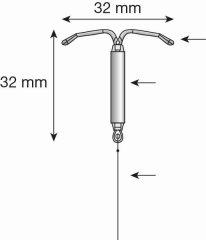
Figure 1b: IUS with Levosert One inserter


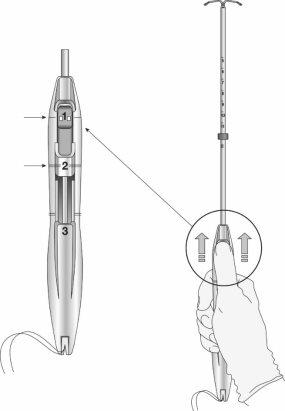
Figure 2: Insertion sliders




Preparation for insertion
Step 1: Open the Levosert One sterile package
- Remove the sealed blister pack containing the device from the box.
- Inspect the sealed blister pack and do not use the product if the packaging, inserter, or IUS is damaged.
- Place the blister pack on a flat surface with the removable cover facing up.
- Remove the removable cover.
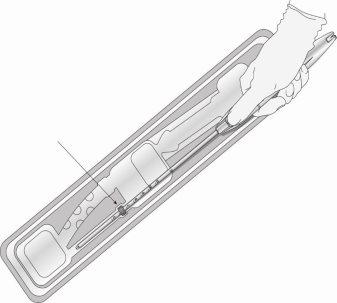
Step 2: Remove the inserter from the blister pack (Figure 3)
Figure 3

Step 3: Slide the sliders completely forward to load the IUS (Figure 4)
Figure 4




Step 4: Load the IUS into the inserter
- Make sure the arms of the IUS are horizontal (aligned with the horizontal plane of the handle and the marker); adjust the rotation of the IUS as necessary using the sterile flat surface of the blister pack.
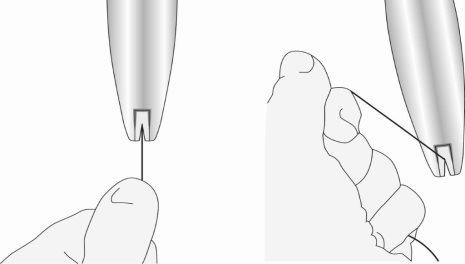
- Maintaining forward pressureon the blue slider, gently pull the threads backwardsto load the IUS into the insertion tube. Make sure to apply uniform tension to both threads when pulling. Pull the threads up or down to lock the threadsin the notch at the base of the handle (Figure 5); you must lock the threads in the notch to prevent the IUS from coming out of the top of the insertion tube. Once the threads are locked in the notch, release them.
Figure 5: Locking the threads in the notch
- Once the IUS is loaded, continue to maintain forward pressure on the blue slider to maintain the correct position of the IUS.
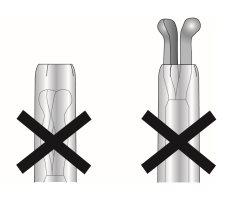
- When it is correctly loaded, the IUS is completely inside the insertion tube with the tips of the arms forming a semispherical cup at the top of the tube (Figure 6, image 1).
Figure 6: Position of the IUS in the insertion tube
Image 1


Image 2

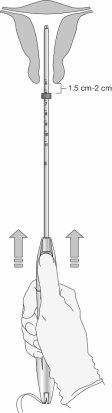
Step 5: Adjust the marker (Figure 7)
Figure 7


Step 6: Insertion of the IUS into the uterus (Figure 8)
Figure 8


Step 7: Release and open the arms of the IUS
Figure 9



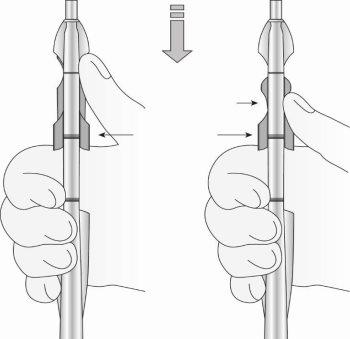
Figure 10: Move the device in the direction of the uterine fundus

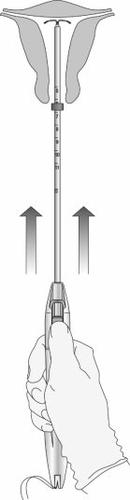
Step 8: Release of the IUS and completion of the procedure
Figure 11: Release of the IUS from the insertion tube


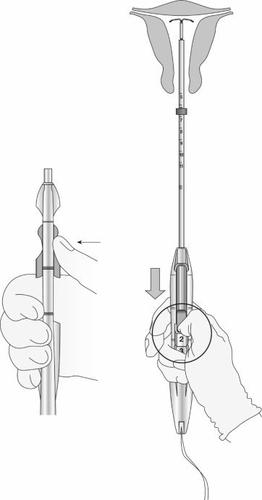
Figure 12: Green indicator visible and threads released from the notch

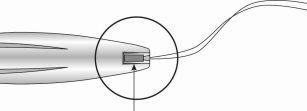
Figure 13: Cut the threads to about 3 cm from the uterine cervix
|
The insertion of Levosert One has been completed.
Important information to consider during or after insertion:
- If you suspect that the IUS is not in the correct position:
- Check the insertion with an ultrasound or other suitable radiological test.
- If incorrect insertion is suspected, remove the IUS. Do not reinsert the same IUS after removal.
IMPORTANT!
In case of a difficult insertion and/or exceptional pain or bleeding during or after insertion, an immediate physical examination and ultrasound should be performed to rule out perforation of the uterine body or cervix. Physical examination alone (including thread checking) may not be sufficient to exclude a partial perforation. If necessary, remove the system and insert a new sterile system.
After insertion, women should be re-examined after 4 to 6 weeks to check the threads and ensure that the device is in the correct position. Report any cases of uterine perforation or insertion difficulties through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es.
Removal/Replacement
The IUS is removed by gently pulling on the threads with forceps. Excessive force or sharp instruments during removal can cause the system to break.
If the threads are not visible and it is discovered that the system is in the uterine cavity during the ultrasound examination, it can be removed using narrow forceps. This may require cervical dilation or surgical intervention.
After removal of the IUS, the system should be examined to ensure that it is intact and that it has been completely removed. During difficult removals, isolated cases have been reported in which the hormone cylinder has slid over the horizontal arms, completely hiding them inside the cylinder. This situation does not require any further intervention once it has been verified that the IUS is complete. The protuberances of the horizontal arms normally prevent the complete separation of the cylinder from the T-shaped body.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LEVOSERT ONE 0.02mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEMDosage form: INTRAUTERINE DEVICE, 13.5 mg levonorgestrelActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 19.5 mgActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 52 mg / initial release rate of 0.02 mg every 24 hoursActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for LEVOSERT ONE 0.02mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM
Discuss questions about LEVOSERT ONE 0.02mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions