
How to use Levosert Easi
Leaflet accompanying the packaging: information for the user
Levosert Easy, 20 micrograms/24 hours, intrauterine therapeutic system
Levonorgestrel
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- If the patient experiences any adverse effects, including any adverse effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Levosert Easy and what is it used for
- 2. Important information before using Levosert Easy
- 3. How to use Levosert Easy
- 4. Possible side effects
- 5. How to store Levosert Easy
- 6. Contents of the packaging and other information
1. What is Levosert Easy and what is it used for
Levosert Easy is an intrauterine therapeutic system (IUS) for use in the uterus, where it gradually releases a hormone called levonorgestrel.
It can be used in the following cases:
Contraceptive method
The Levosert Easy system is an effective, long-term, and temporary (reversible) contraceptive method.
The Levosert Easy system prevents pregnancy by inhibiting the growth of the uterine lining, thickening the cervical mucus (in the cervical canal), which prevents sperm from entering the egg cell and inhibiting ovulation in some women.
It may also have a local effect on the uterine lining caused by the presence of the T-shaped intrauterine system.
Treatment of excessive menstrual bleeding
The Levosert Easy system is also used to reduce the heaviness of menstrual bleeding and can be used to treat heavy menstrual bleeding (periods), known as menorrhagia.
The hormone contained in the Levosert Easy system acts to inhibit the monthly development of the uterine lining, so that less bleeding occurs each month.
The Levosert Easy system is effective for 8 years as a contraceptive indication (preventing pregnancy) and for 5 years as an indication for excessive menstrual bleeding.
To ensure contraception, the Levosert Easy system should be removed before the end of the eighth year and replaced with a new Levosert Easy system immediately if further use is required.
No additional protection is required in the case of immediate insertion of the system.
In the case of the indication for excessive menstrual bleeding, the Levosert Easy system should be replaced before the end of the fifth year.
If symptoms have not returned by the end of the fifth year of use, further use after five years may be considered.
The system should be removed or replaced no later than after 8 years.
Children and adolescents
The Levosert Easy system is not indicated for use before the first menstrual period (menarche).
2. Important information before using Levosert Easy
Before inserting the Levosert Easy system, the doctor or nurse may order several tests to ensure that the Levosert Easy system is suitable for the patient to use.
This includes a pelvic examination, but other tests, such as a breast examination, may also be performed if the doctor or nurse considers it appropriate.
Before inserting the Levosert Easy system, patients must be effectively cured of genital infections.
The doctor or nurse should be informed if the patient has epilepsy, as a seizure may occur, although rarely, during the insertion of the Levosert Easy system.
Some women may feel weak after the procedure.
This is a normal condition, and the doctor or nurse will inform the patient to rest for a while.
Not all women can use the Levosert Easy intrauterine system.
The Levosert Easy intrauterine system should not be used if the patient:
- is pregnant or suspects pregnancy;
- has current or past inflammatory conditions of the pelvic organs;
- has abnormal or unpleasant vaginal discharge or vaginal itching, which may indicate an infection;
- has had an infection of the uterine lining after childbirth;
- has had uterine infections after childbirth or miscarriage within the last 3 months;
- has had cervical inflammation;
- has had abnormal cervical cytology test results (changes in the cervix);
- has liver tumors or other acute or severe liver diseases;
- has uterine abnormalities, including uterine fibroids, especially if they distort the uterine cavity;
- has abnormal vaginal bleeding;
- has any disease that makes the patient prone to infections.
The doctor will inform the patient if such a situation applies to them; - has had a hormone-dependent tumor, such as breast cancer;
- has had any type of tumor or suspected tumor, including blood cancer (leukemia), cervical cancer, or uterine cancer, unless it is in remission;
- has had trophoblastic diseases.
The doctor will inform the patient if such a situation applies to them; - is allergic to levonorgestrel or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
The Levosert Easy system, like other hormonal contraceptives, does not protect against HIV infection (AIDS) or other sexually transmitted diseases (such as chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B virus, and syphilis).
Only the use of condoms can provide protection against these diseases.
The Levosert Easy system should not be used as emergency contraception (after unprotected sex).
Before starting to use the Levosert Easy system, the patient should discuss it with their doctor if:
- -the patient has migraines, dizziness, vision disturbances, exceptionally severe headaches, or more frequent headaches than usual;
- the patient has jaundice (yellowing of the skin and whites of the eyes);
- the patient has high blood pressure;
- the patient has diabetes (high blood sugar levels) or abnormal blood lipid levels;
- the patient has had blood cancer (including leukemia) that is currently in remission;
- the patient is taking long-term steroid therapy;
- the patient has had an ectopic pregnancy (fetal development outside the uterus) or ovarian cysts;
- the patient has had severe arterial disease, such as a heart attack or stroke, or has any heart problems;
- the patient has had blood clots (thrombosis) in the past;
- the patient is taking any other medicines, as some medicines may interfere with the action of the Levosert Easy system;
- the patient has irregular menstrual bleeding;
- the patient has epilepsy (seizures).
If any of the above conditions occur or have occurred, the doctor will decide whether the Levosert Easy system can be used.
If the patient is using the Levosert Easy system and any of the above conditions occur for the first time, the doctor should also be informed.
The following subjective and objective symptoms may indicate an ectopic pregnancy (a pregnancy developing outside the uterus) and, in such a case, the patient should immediately consult a doctor (see also the section "Pregnancy, breastfeeding, and fertility"):
- Menstrual bleeding has stopped, and then persistent bleeding or pain has occurred.
- Severe or persistent abdominal pain has occurred.
- The patient has experienced normal pregnancy symptoms, but also bleeding and dizziness.
- A pregnancy test is positive.
If the patient experiences painful swelling of the leg, sudden chest pain, or difficulty breathing, they should see a doctor or nurse as soon as possible, as these may be symptoms of a blood clot.
It is essential that any blood clots are treated as soon as possible.
The patient should also consult a doctor immediately if they experience persistent abdominal pain, fever, pain during sex, or abnormal bleeding.
If the patient experiences sudden pain or fever soon after the insertion of the Levosert Easy system, it may mean that the patient has a severe infection that needs to be treated immediately.
Expulsion
The uterine contractions during menstrual bleeding may sometimes cause the system to move or be expelled.
This is more likely if the woman is overweight at the time of insertion or if she has had heavy menstrual bleeding in the past.
If the system is not in place, it may not work as intended, and the risk of pregnancy increases.
Expulsion of the system results in loss of protection against pregnancy.
Possible symptoms of expulsion include vaginal bleeding or abdominal pain, but the Levosert Easy system can also be expelled without the patient noticing.
Since the Levosert Easy system reduces menstrual bleeding, the intensity of menstrual bleeding may be a sign of expulsion or displacement of the system.
It is recommended to check by feeling with the fingers (e.g., during bathing) whether the threads are in the correct position.
See also section 3 "How to use Levosert Easy - Self-checking of the correct position of the Levosert Easy system".
If symptoms suggesting expulsion of the system occur or the threads cannot be felt in the cervical area, the patient should use other contraceptive methods (such as condoms) and consult a doctor.
Mental disorders
Some women using hormonal contraceptives, including Levosert Easy, have reported depression or low mood.
Depression can be severe and sometimes lead to suicidal thoughts.
If mood changes and symptoms of depression occur, the patient should consult a doctor as soon as possible to receive further medical advice.
Levosert Easy and smoking
Women should quit smoking.
Smoking increases the risk of heart attack, stroke, or blood clots.
Using tampons and menstrual cups
It is recommended to use sanitary pads.
If tampons or menstrual cups are used, they should be changed carefully to avoid pulling on the Levosert Easy system threads.
Levosert Easy and other medicines
Since the mechanism of action of the Levosert Easy system is mainly local, taking other medicines should not increase the risk of pregnancy during the use of the Levosert Easy system.
However, it is recommended that the patient tells their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those available without a prescription.
This includes the following medicines:
- medicines used to treat epilepsy (such as lamotrigine, barbiturates, phenytoin, primidone, carbamazepine, oxcarbazepine, topiramate, felbamate)
- medicines used to treat tuberculosis (such as rifampicin, rifabutin)
- medicines used to treat HIV and hepatitis C virus infections (such as nevirapine, efavirenz, ritonavir, nelfinavir)
- medicines used to treat fungal infections (such as griseofulvin, itraconazole, ketoconazole)
- medicines used to treat bacterial infections (such as clarithromycin, erythromycin)
- medicines used to treat certain heart conditions, high blood pressure (such as verapamil, diltiazem)
- herbal medicines containing St. John's Wort (Hypericum perforatum)
- grapefruit juice
The Levosert Easy system should not be used at the same time as other hormonal contraceptive methods.
Pregnancy, breastfeeding, and fertility
The Levosert Easy system should not be used during pregnancy or if pregnancy is suspected.
Can the patient become pregnant while using the Levosert Easy system?
Women using the Levosert Easy system very rarely become pregnant.
Lack of menstrual bleeding does not necessarily mean that the woman is pregnant.
In some women, menstrual bleeding may not occur during the use of the intrauterine system.
If menstrual bleeding has not occurred for 6 weeks, a pregnancy test should be considered.
If the result is negative, there is no need to perform another test unless other symptoms suggesting pregnancy occur, such as nausea, fatigue, and breast tenderness.
If the woman becomes pregnant with the Levosert Easy system in place, she should immediately consult a doctor to have the Levosert Easy system removed.
Removal may cause a miscarriage.
However, leaving the Levosert Easy system in place during pregnancy may increase not only the risk of miscarriage but also the risk of preterm birth.
If the Levosert Easy system cannot be removed, the patient should discuss the benefits and risks of continuing the pregnancy with their doctor.
If the pregnancy continues, it should be monitored closely by the doctor, and the patient should immediately inform their doctor if they experience symptoms such as abdominal cramps, abdominal pain, or fever.
Levosert Easy contains a hormone called levonorgestrel, and there have been single reports of its effect on the genital organs of girls exposed to levonorgestrel released from an intrauterine device left in the uterus.
What should the patient do if they want to have a child?
If the patient wants to become pregnant and have a child, they should ask their doctor to remove the Levosert Easy system.
After removal of the system, natural fertility returns soon.
Can the patient breastfeed while using the Levosert Easy system?
Very small amounts of the hormone in the Levosert Easy system have been found in breast milk, but the levels are lower than with any other hormonal contraceptive method.
There is no need to worry about the risk to the newborn.
If the woman wants to breastfeed, she should discuss it with her doctor.
Driving and using machines
The effect on the ability to drive and use machines is not known.
Levosert Easy contains barium sulfate.
The T-shaped frame of the Levosert Easy system contains barium sulfate, so it may be visible on X-ray images.
3. How to use Levosert Easy
The system should only be inserted by a doctor or a specially trained nurse (see the special insertion instructions included in the packaging).
These individuals will explain the insertion procedure and any risks associated with its use.
Before inserting the Levosert Easy system, the patient will be examined by a doctor or nurse.
If the patient has any concerns about using the system, they should discuss them with their doctor or nurse.
During the insertion procedure, the patient may experience some discomfort.
The patient should inform their doctor about any pain they feel.
Starting to use the Levosert Easy system
- Before inserting the Levosert Easy system, it should be ensured that the patient is not pregnant.
- The Levosert Easy system should be inserted within 7 days of the start of menstruation.
If the Levosert Easy system is inserted during menstruation or within 7 days of its start, the Levosert Easy system will work immediately and prevent pregnancy. - If the Levosert Easy system cannot be inserted within 7 days of the start of menstruation or if menstruation occurs at an unpredictable time, the Levosert Easy system can be inserted on any other day.
In this case, the patient should not have unprotected sex and should use additional contraceptive protection for 7 days before insertion, and a negative pregnancy test result should be obtained before insertion.
Additionally, the Levosert Easy system may not prevent pregnancy immediately. - The Levosert Easy system is not intended for use as emergency contraception (after unprotected sex).
Starting to use the Levosert Easy system after childbirth
- The Levosert Easy system can be inserted after childbirth, when the uterus has returned to its normal size, but no earlier than 6 weeks after childbirth (see section 4 "Possible side effects - Perforation").
- More information on the timing of insertion can be found in the "Starting to use Levosert Easy" section above.
Starting to use the Levosert Easy system after a miscarriage
The Levosert Easy system can be inserted immediately after a miscarriage, if the pregnancy lasted less than 3 months, provided that there are no genital infections.
The Levosert Easy system will be effective immediately.
Replacing the Levosert Easy system
The Levosert Easy system can be replaced with a new Levosert Easy system at any time during the menstrual cycle.
The Levosert Easy system will be effective immediately.
Switching from another contraceptive method (such as combined hormonal contraceptives, implant)
- The Levosert Easy system can be inserted immediately if it is certain that the patient is not pregnant.
- If more than 7 days have passed since the last menstrual period, the patient should abstain from sex or use additional contraceptive protection for 7 days.
Inserting the Levosert Easy system
The examination by a healthcare professional before inserting the system may include:
- a cervical smear (cytological smear)
- a breast examination
- other tests, if necessary, such as tests for infections, including sexually transmitted diseases, and a pregnancy test.
The healthcare professional will also perform a gynecological examination to determine the position and size of the uterus.
After the gynecological examination
- A device called a speculum is inserted into the vagina, and the cervix may be cleaned with an antiseptic solution.
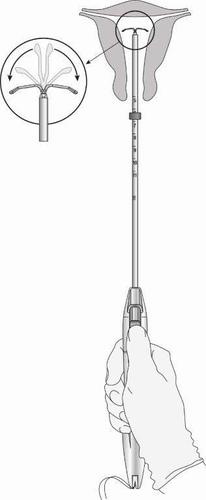
The Levosert Easy system is then placed in the uterus using a thin, flexible plastic tube (insertion tube).
Local anesthesia may be applied before inserting the tube into the cervix. - Some women may feel dizzy or faint during or after the insertion of the Levosert Easy system, or after its removal.
- Pain and bleeding may occur during or immediately after insertion.
After the insertion of the Levosert Easy system, the patient should receive a reminder card from their doctor for follow-up examinations.
The patient should bring this card to each scheduled follow-up visit.
After the insertion of the Levosert Easy system, the patient should receive a reminder card from their doctor for follow-up examinations.
The patient should bring the reminder card to each scheduled follow-up visit.
How soon will the Levosert Easy system start working?
Contraception
If the Levosert Easy system is inserted into the uterus during menstruation or within 7 days of its start, or if the patient already has a system in place and it is time to replace it with a new one, or if the patient has just had a miscarriage, the patient is protected against pregnancy from the moment the intrauterine system is inserted.
The likelihood of pregnancy is about 2 cases per 1000 women in the first year of using the system.
Emergency situations may increase if the Levosert Easy system is expelled or perforates.
Excessive menstrual bleeding
After using the Levosert Easy system, menstrual bleeding usually decreases significantly within 3 to 6 months of therapy.
How often should the system be checked?
A check of the Levosert Easy system should be performed 4 to 6 weeks after insertion, and then regularly, at least once a year, until it is removed.
The doctor may determine how often and what type of checks are required in each case.
The patient should bring the reminder card they received from their doctor to each scheduled follow-up visit.
Additionally, the patient should consult a doctor if any of the symptoms described in section 2 "Warnings and precautions" occur.
Additionally, the patient should see a doctor as soon as possible if they experience:
- painful swelling of the leg,
- sudden chest pain,
- difficulty breathing, as these may be symptoms of a blood clot.
How can it be determined if the system is in place?
After each menstrual period, the woman can check for the presence of two threads attached to the lower edge of the system.
The doctor will show her how to do this.
Do not pullon the threads, as this may cause the system to be accidentally removed.
If the woman cannot find the threads, she should see a doctor or nurse as soon as possible, and in the meantime, she should not have unprotected sex or use barrier contraception (such as condoms).
The threads may have moved up into the uterus or cervix.
If the threads are still not found by the doctor or nurse, they may have broken off, or the Levosert Easy system may have been expelled or perforated the uterine wall (uterine perforation, see section 4).
The patient should also see a doctor if they can feel the lower edge of the system or if they or their partner experience pain or discomfort during sex.
What happens if the system is expelled?
If the system is expelled, either completely or partially, it will not provide protection against pregnancy.
Expulsion of the system can occur, although rarely, during menstruation, without the patient noticing.
A possible sign may be an unusual increase in menstrual bleeding.
The patient should inform their doctor or clinic staff if they experience any unexpected changes in their bleeding pattern.
Removing the Levosert Easy system
The Levosert Easy system should be removed or replaced after 8 years of use for contraception and after 5 years of use for excessive menstrual bleeding, or earlier if heavy or troublesome menstrual bleeding returns.
If symptoms have not returned by the end of the fifth year of use, further use after five years may be considered.
The system should be removed or replaced no later than after 8 years.
The doctor can easily remove the system at any time, after which the patient can become pregnant.
Some women may feel dizzy or faint during or after the removal of the Levosert Easy system.
Pain and bleeding may occur during the removal of the Levosert Easy system.
Continuing contraception after removal of the system
If the patient does not plan to become pregnant, the Levosert Easy system should not be removed after the seventh day of the menstrual cycle (menstruation), unless the patient is using other contraceptive methods (e.g., condoms) for at least 7 days before removal.
In the case of irregular periods or amenorrhea, the patient should use barrier contraception for 7 days before removal.
A new Levosert Easy system can also be inserted immediately after removal of the previous one, in which case additional protection is not necessary.
If the patient does not want to continue using the same method, they should ask their doctor for advice on other effective contraceptive methods.
How does the Levosert Easy system affect menstrual bleeding?
For all patients using the Levosert Easy system
Many women experience spotting (small amounts of blood) during the first 3-6 months after insertion of the system.
In some women, menstrual bleeding may be prolonged or heavier.
The patient may experience heavier bleeding, usually during the first 2 to 3 months, before the reduction in blood loss occurs.
It is possible that the number of days of menstrual bleeding will decrease over time, and eventually, menstrual bleeding may not occur at all.
This is due to the effect of the hormone (levonorgestrel) on the uterine lining.
If the patient has the Levosert Easy system inserted to reduce excessive menstrual bleeding
The Levosert Easy system usually significantly reduces the heaviness of menstrual bleeding within 3 to 6 months of treatment.
However, before the heaviness of bleeding is reduced, during the first 2 to 3 months of treatment, bleeding may be heavier.
If there is no significant reduction in bleeding after 3 to 6 months, other treatment methods should be considered.
If the Levosert Easy system has been in place for some time and bleeding disturbances have occurred, the patient should consult a doctor or other healthcare professional.
If the patient has any questions about using this medicine, they should consult their doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects of the Levosert Easy system most commonly occur during the first few months after insertion of the system and decrease over time.
In case of any of the following serious side effects, the patient should immediately consult a doctor or nurse:
- Severe pain or fever occurring soon after insertion of the systemmay indicate that the patient has developed a severe infection that needs to be treated immediately.
In rare cases, a very severe infection (sepsis) may occur. - Severe pain and persistent bleeding, as this may be a sign of uterine perforation.
Perforation is not very common but usually occurs during the insertion of the Levosert Easy system, although it may only be diagnosed later.
A Levosert Easy system that is located outside the uterine cavity is not effective in preventing pregnancy and should be removed as soon as possible; very rarely, this may require surgery.
The risk of perforation is low but may be increased in breastfeeding women and in women who have given birth to a child less than 36 weeks before insertion of the system, and the risk may also be increased in women with a permanently retroverted uterus (retroversion of the uterus).
If the patient suspects uterine perforation, they should see their doctor immediately and inform them that they have a Levosert Easy system in place, especially if it is not the doctor who inserted the system.
Possible objective and subjective symptoms of perforation may include: - severe pain (resembling menstrual cramps) or more severe pain than expected,
- heavy bleeding (occurring after insertion of the system),
- pain or bleeding lasting longer than a few weeks,
- sudden changes in menstrual bleeding,
- pain during sex,
- the patient cannot find the threads of the Levosert Easy system (see section 3 "How to use Levosert Easy - How to check if the system is in place?").
- Abdominal pain, especially if the patient has a fever or has not had menstrual bleeding or has had unexpected bleeding, as this may indicate an ectopic pregnancy.
The overall risk of ectopic pregnancy in women using the Levosert Easy system is small.
However, if a woman becomes pregnant while using the Levosert Easy system, the relative likelihood of an ectopic pregnancy increases. - Abdominal pain or pain during sex, as this may indicate an ovarian cyst or pelvic inflammatory disease.
This is important because pelvic infections can reduce the chances of becoming pregnant and can increase the risk of ectopic pregnancy.
Very common(may affect more than 1 in 10 women) side effects:
- changes in menstrual bleeding.
Spotting, shorter or longer menstrual bleeding, or painful menstruation may occur.
Although the Levosert Easy system usually results in a significant reduction in menstrual blood loss within 3 to 6 months of therapy, during the first 2 to 3 months, bleeding may be heavier before the reduction in blood loss occurs.
Menstrual bleeding may completely stop.
If there is no significant reduction in blood loss within 3 to 6 months, other treatment methods should be considered; - ovarian cysts.
These are fluid-filled sacs in the ovary; - bacterial or fungal infections of the vagina or external genitalia (vulva);
- acne (pimples);
- pain or bleeding during insertion of the system.
Common(affecting up to 1 in 10 women) side effects:
- depression, nervousness, or other mood changes;
- reduced libido;
- headaches;
- migraines;
- feeling weak (pre-syncope);
- dizziness;
- abdominal, pelvic, or back pain;
- discomfort in the abdominal cavity;
- nausea;
- bloating;
- vomiting;
- painful menstruation;
- excessive vaginal discharge;
- vulvovaginitis (inflammation of the vulva and vagina);
- breast tenderness or breast pain;
- pain during sex;
- uterine cramps;
- expulsion of the intrauterine system;
- weight gain.
Uncommon(affecting up to 1 in 100 women) side effects:
- genital infections, which can cause: vaginal itching, pain when urinating, or abdominal pain (lower abdominal pain) associated with inflammation of the uterus, ovaries, or fallopian tubes;
- fainting;
- hives;
- cervical inflammation;
- swelling or edema of the legs or ankles;
- increased hair growth on the face and body;
- hair loss;
- skin itching (pruritus);
- skin discoloration or increased pigmentation, especially on the face (chloasma);
- ectopic pregnancy;
- perforation (puncture) of the uterine wall (see above "serious side effects").
Rare(affecting up to 1 in 1000 women) side effects:
- rash, itching.
Severe pain or fever occurring soon after insertion of the system may indicate that the patient has developed a severe infection that needs to be treated immediately.
In rare cases, a very severe infection (sepsis) may occur.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should inform their doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, it is possible to gather more information on the safety of the medicine.
5. How to store Levosert Easy
Store in the original packaging.
There are no special storage temperature recommendations for the medicine.
Store the sealed tray in the outer packaging to protect it from light.
Keep the packaging tightly closed.
The sealed tray should only be opened by a doctor or clinic staff.
Medicines should be kept out of the sight and reach of children.
Do not use the system after the expiry date stated on the label and outer packaging after "EXP".
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste.
The patient should ask their pharmacist how to dispose of medicines that are no longer required.
This will help protect the environment.
6. Contents of the packaging and other information
What Levosert Easy contains
- The active substance of the medicine is 52 mg of levonorgestrel.
The hormone is placed in a substance called polydimethylsiloxane (PDMS).
This is surrounded by a membrane, which is also made of polydimethylsiloxane (PDMS).
What Levosert Easy looks like and what the packaging contains
- Levosert Easy consists of a small T-shaped frame made of LDPE with an added 20-24% barium sulfate.
This design of the system allows for the gradual release of the hormone into the uterine cavity. - The system also contains two thin threads made of PE, dyed with copper phthalocyanine, blue.
The threads are attached to the lower part of the frame.
They allow the doctor to easily remove the system and check that the system is in the correct position.
Levosert Easy, the intrauterine therapeutic system with an insertion device, is packaged in a thermoformed, transparent, plastic tray with a removable lid made of HDPE.
The sterile tray is packaged in a cardboard box with a patient information leaflet and a patient reminder card.
Pack sizes:
The packaging contains one intrauterine therapeutic system.
A multipack contains 5 packagings.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Gedeon Richter Polska Sp. z o.o.
ul. Ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
Manufacturer
Odyssea Pharma SRL
Rue du Travail 16
4460 Grâce-Hollogne
Belgium
Gedeon Richter Plc.
Gyömrői út 19-21.
1103 Budapest
Hungary
To obtain more detailed information on the medicine, the patient should contact:
Gedeon Richter Polska Sp. z o.o.
Medical Department
ul. ks. J. Poniatowskiego 5
05-825 Grodzisk Mazowiecki
phone: +48 (22)755 96 48
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Hungary
Levosert Single-Handed Inserter
Bulgaria
Донасертон 20 микрограма/24 часа вътрематочна лекарстводоставяща система
Donasertone 20 micrograms/24 hours Intrauterine Delivery System
Czech Republic
Levosert SHI
Latvia
Levosert SHI 20 mikrogramu/24 stundās intrauterīna sistēma
Lithuania
Levosert Single-Handed Inserter 20 mikrogramų/24 valandų vartojimo į gimdos ertmę sistema
Poland
Levosert Easy
Romania
Donasertone 20 micrograme/24 ore sistem cu cedare intrauterină
Slovakia
Levosert SHI
Date of last revision of the leaflet: November 2024
The following information is intended for healthcare professionals only
See: special instructions included in the packaging
Instructions for use and storage
Levosert Easy 20 micrograms/24 hours intrauterine therapeutic system
Levonorgestrel
Checklist for the prescribing doctor
Before prescribing and/or inserting the Levosert Easy system, the doctor should ensure that:
- The patient meets all the indications for adjustment of contraception or has excessive menstrual bleeding and also meets the criteria for the duration of treatment, which is eight or five years.
- The patient card included in the packaging has been completed and given to the patient (each case of using the system for longer than eight years for contraception and/or for the treatment of excessive menstrual bleeding should be reported as off-label use).
Instructions for inserting the system
The doctor should carefully read the following instructions, as there may be differences in the type of system compared to other intrauterine devices used previously:
Before Insertion by a Healthcare Provider Using the Aseptic Technique
It is recommended that the Levosert Easy system be inserted only by healthcare providers with experience in inserting an intrauterine system (IUS) and/or who have received appropriate training on the insertion of the Levosert Easy system and have carefully reviewed this instruction before inserting the Levosert Easy system.
The Levosert Easy system is provided in a sterile package, which should not be opened until the time of use. It should not be resterilized. The system can only be used once.
The unpackaged system should be inserted under aseptic conditions. If the sterile package is damaged, the system should be discarded (see section 6.6 of the Summary of Product Characteristics). It should not be used if the inner package is damaged or opened. It should not be used after the expiration date stated on the carton and blister pack after EXP. The expiration date indicates the last day of the given month.
More information on the insertion of the intrauterine system can be found in the Summary of Product Characteristics, section 4.2.
The Levosert Easy system is provided with a patient reminder card for control examinations included in the outer package. The patient reminder card should be completed and given to the patient after the system has been inserted.
Preparation for Insertion of the System
- The patient should be examined to rule out contraindications for the insertion of the Levosert Easy system (see sections 4.3 and 4.4 in the section entitled "Medical Examination").
- A speculum should be inserted, the cervix visualized, and then the cervix and vagina cleaned with an appropriate antiseptic solution.
- If necessary, an assistant may be used.
- The anterior lip of the cervix should be grasped with a surgical forceps or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip of the cervix. To straighten the cervical canal, the cervix may be gently pulled with the forceps. The forceps should remain in place, and gentle traction on the cervix should be maintained throughout the insertion procedure.
- A sound should be inserted through the cervical canal to the uterine fundus to measure the depth. If the uterine depth is less than 5.5 cm, the insertion should be discontinued. The position of the uterine cavity and any abnormalities within the uterine cavity (e.g., septum, submucous fibroids) or a previously inserted intrauterine contraceptive that has not been removed should be confirmed. If difficulties are encountered, consideration should be given to dilating the cervical canal. If dilation of the cervix is necessary, consideration should be given to using analgesics and/or cervical block.
Description
Figure 1a: Levosert Easy Intrauterine System
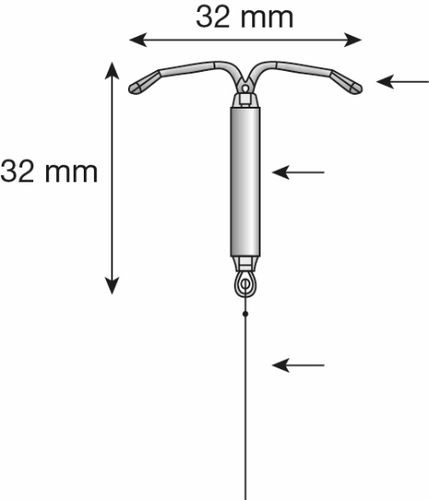
Plastic frame in the shape of the letter T
Hormone reservoir with a membrane
Blue threads for removing the system
Figure 1b: Levosert Easy Intrauterine System with Applicator
Levosert Easy intrauterine system

Insertion tube
Collar
BLUE slider
GREEN slider
Handle
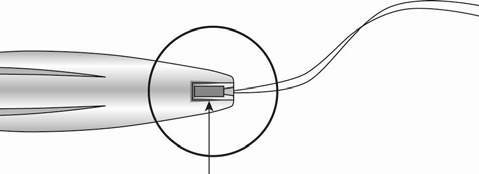
Slot
Threads
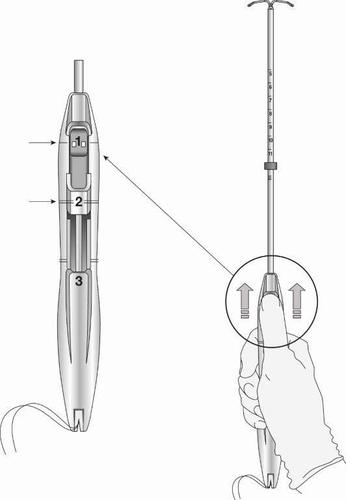
The Levosert Easy intrauterine system is partially loaded in the applicator. The threads pass through the insertion tube and exit through the slot opening in the handle.
In the applicator handle, there is a BLUE slider marked with the number 1 and a GREEN slider marked with the number 2. The handle is marked with the number 3. The sliders are marked with numbers 1 and 2, and the handle is marked with the number 3, which helps in the insertion process (Figure 2).
Moving the sliders allows achieving the position required to complete the insertion process.
Figure 2: Applicator Sliders

NUMBER 1
NUMBER 2
- In the applicator handle, there is a BLUE slider marked with the number 1 and a GREEN slider marked with the number 2, which helps in the insertion process.
- Moving the sliders allows achieving the position required to complete the insertion process.
Preparation for Insertion of the System
Open the Sterile Package of the Levosert Easy System
- Remove the sealed inner package containing the Levosert Easy system from the box.
- Check the seal of the inner package; if the inner package, applicator, or intrauterine system is damaged, do not use the product.
- Place the inner package on a flat surface, with the removable top facing up.
- Remove the top.
Loading the Levosert Easy System into the Applicator
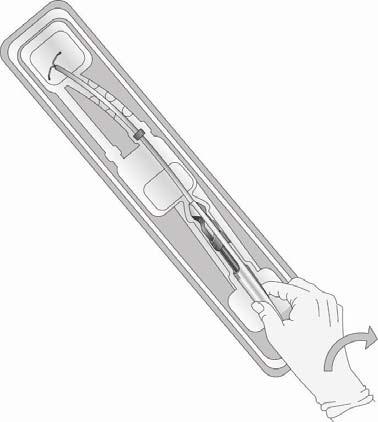
- To remove the applicator from the inner package, grasp the handle below the sliders and gently twist (Figure 3).
- WARNING: Do not remove the applicator by pulling on the insertion tube.
Figure 3: Removing the Applicator from the Inner Package
Make sure that both sliders (marked with numbers 1 and 2) are completely advanced
(Figure 4):
Figure 4: Sliders Completely Advanced, Ready to Load the Levosert Easy System
NUMBER 1
NUMBER 2
- The BLUE slider (marked with number 1) has a single-line marking, which will align with the single-line marking on the handle.
- The GREEN slider (marked with number 2) has a double-line marking, which will align with the double-line marking on the handle.
- Grasp the handle, holding the thumb or finger in the depression of the BLUE slider (above
number 1) and push forward, ensuring that both sliders are completely advanced.
Loading the Levosert Easy System into the Applicator:
- Make sure that the arms of the intrauterine system are in a horizontal position (aligned with the horizontal plane of the handle and collar); if necessary, adjust the rotation of the intrauterine system using the flat, sterile surface of the inner package.
- While maintaining forward pressureon the blue slider, gently pull the threads straightback to load the Levosert Easy system into the insertion tube. Ensure that both threads are evenly tensioned during pulling and pull them up or down to lock the threads in the slot, in the lower part of the handle (Figure 5); lock the threadsin the slot to prevent the intrauterine system from advancing out of the upper part of the insertion tube. Once the threads are locked in the slot, release the threads.
Figure 5: Locking the Threads in the Slot
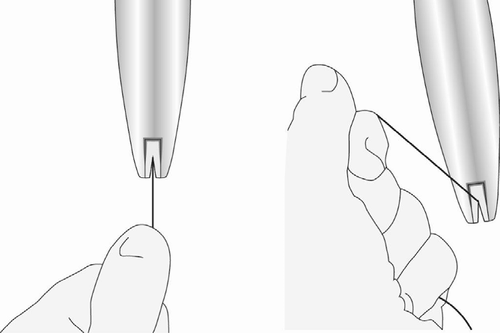
- After loading the intrauterine system, maintain forward pressureon the BLUE slider to maintain the hemispherical bulge formed by the ends of the arms of the intrauterine system.
- After proper loading of the intrauterine system, it is completely in the insertion tube, and the ends of the arms form a hemispherical bulge in the upper part of the insertion tube (Figure 6).
Figure 6: Close-up of the Hemispherical Bulge at the End of the Insertion Tube
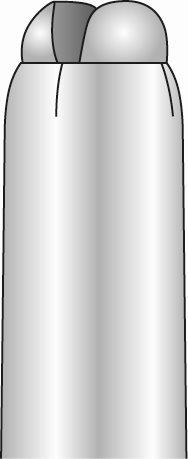
- If the intrauterine system is not properly loaded, do not attemptinsertion.
- To reload the Levosert Easy system:
- Pull the thumb on the BLUE slider back until the depression aligns with the GREEN slider to release the intrauterine system.
- Manually pull the threads out of the slot.
- Move the BLUE slider forward and repeat the loading process.
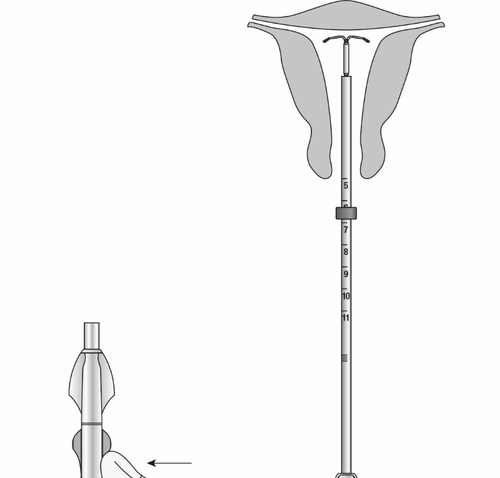
- Adjust the collar to the measured uterine depth based on sounding. To adjust the collar, place the flat side of the collar in the cutout of the inner package (Figure 7) or against a sterile edge inside the package. If necessary, move the insertion tube to move the collar to the correct position. Ensure that the flat sides of the collar are in the same horizontal plane as the handle. If at
any stage it becomes necessary to touch the collar or other sterile surface, use sterile gloves.
Figure 7: Adjusting the Collar
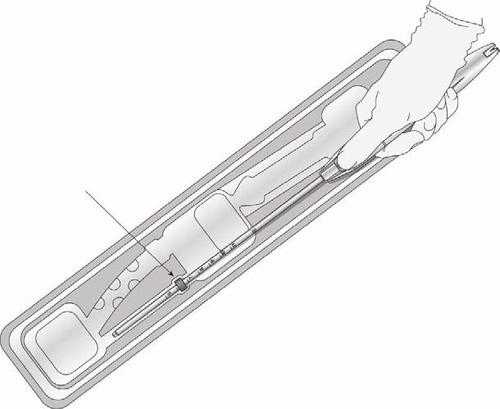
Cutout in the inner
package
- If it is necessary to correct the curvature of the insertion tube to adapt to the anatomical position of the uterus, the insertion tube can be bent or straightened without touching it above the collar, unless using sterile gloves. When bending the tube, avoid sharp bends to prevent it from breaking.
- After properly positioning the collar, avoid contact between the collar and objects that may change its position (e.g., inner package, speculum, forceps, etc.).
Insertion of the Levosert Easy System into the Uterus
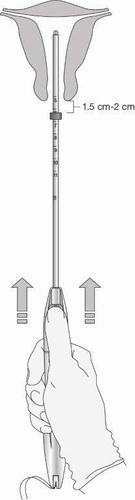
- Gently pull the forceps and continue to apply forward pressureon the BLUE slider while inserting the loaded insertion tube through the cervical os. Advance the tube until the upper edge of the collar is 1.5-2 cm from the external cervical os (Figure 8). Maintain forward pressure on the BLUE slider throughout the insertion process.
- At this point, DO NOT advance the collar toward the cervix.
- DO NOT force the applicator. If necessary, dilate the cervical canal.
Figure 8: Advancing the Insertion Tube until the Collar is 1.5-2 cm from the Cervical Os
- Using the thumb or finger, gently move the BLUE slider back. Initially, a slight resistance is felt before moving the BLUE slider from its initial position. Continue to move the BLUE slider back until a slight resistance is felt again, as the BLUE and GREEN sliders engage, forming a common depression. Do not move the BLUE slider further than necessary to form the depression. Maintain the GREEN slider so that the double-line markings on the slider and handle remain aligned (Figure 9). This will allow the arms of the intrauterine system to open in the lower part of the uterus. Do not pull the sliders back further, as this may cause premature release of the intrauterine system in the wrong location.
Figure 9: Releasing and Opening the Arms of the Intrauterine System
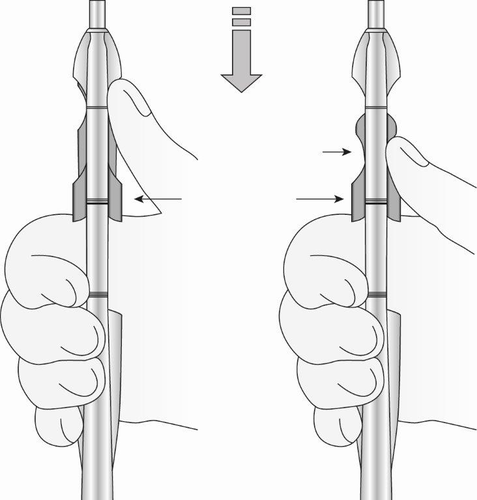
- Wait 10-15 seconds to allow the arms of the intrauterine system to fully open.
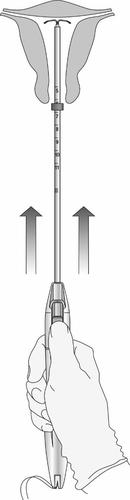
- Without moving the sliders, advance the applicator until the collar touches the cervix. If
resistance is encountered, do not continue to advance. The Levosert Easy system is now in the correct position (Figure 10).
- Warning: Proper positioning of the intrauterine system within the uterus is essential to preventexpulsion.
Figure 10: Advancing the Levosert Easy System to the Uterine Fundus
Releasing the Levosert Easy System and Completing the Insertion Procedure
- Holding the applicator steady, maintaining its position relative to the cervix, move both sliders (BLUE and GREEN) down together, maintaining the common depression of the slider in the direction of number 3 on the handle (Figure 11), until a click is heard and the GREEN marker in the lower part of the handle is visible (Figure 12).
Figure 11: Releasing the Levosert Easy System from the Insertion Tube
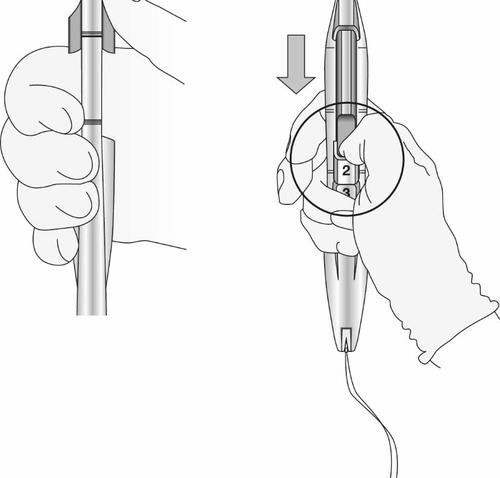
- Look at the slot to ensure that the threads have been properly released (Figure 12); if they have not been released or if a click was not heard, grasp the threads and gently pull them out of the slot.
Figure 12: Visible Green Marker and Released Threads from the Slot
- Remove the applicator from the uterus.
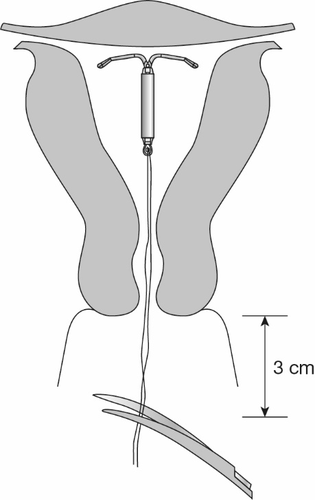
- Using blunt-tipped sharp scissors, cut the intrauterine threads perpendicular to the thread position, leaving about 3 cm outside the cervix (Figure 13). Note: Do not cut the threads at an angle, as this may leave sharp ends.
- When cutting, do not pull or tug on the threads to prevent moving the intrauterine system.
Figure 13: Cutting the Threads about 3 cm from the Cervix
The insertion procedure of the Levosert Easy intrauterine system is complete.
Important information to consider during or after insertion:
- If there is suspicion that the intrauterine system is not in the correct position:
- Check the position of the system using ultrasound or another appropriate radiologic test.
- If abnormal positioning is suspected, the Levosert Easy intrauterine system should be removed. Do not reinsert the same Levosert Easy intrauterine system after its removal.
IMPORTANT!
In case of difficulties during insertion and/or severe pain or bleeding during or after insertion, consider the possibility of perforation and take appropriate measures, such as physical and ultrasonographic examination. If necessary, remove the system and insert a new, sterile one.
After insertion of the system, patients should be re-examined after 4 to 6 weeks to check the threads and ensure that the system is in the correct position. A physical examination alone (including thread check) may not be sufficient to rule out partial perforation.
Any cases of uterine perforation or difficulties with system insertion should be reported through the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Removal/Replacement
To remove the Levosert Easy system, gently pull on the threads with forceps. If the threads are not visible and the system has been detected in the uterus during ultrasonography, narrow forceps may be used. This method may require dilation of the cervical canal or surgical intervention. After removing the Levosert Easy system, check that the system has not been damaged.
In particularly difficult removal procedures, isolated cases have been reported of the hormone cylinder being displaced above the horizontal arms and hiding them together inside the cylinder. This situation does not require further intervention, provided that the intrauterine system (IUS) remains complete. The bulges of the horizontal arms usually prevent the complete separation of the cylinder from the T-shaped scaffold.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGedeon Richter Plc. Odyssea Pharma SA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Levosert EasiDosage form: System, 19.5 mg/systemActive substance: plastic IUD with progestogenManufacturer: BAYER OyPrescription requiredDosage form: System, 52 mg (20 mcg/24 h)Active substance: plastic IUD with progestogenPrescription requiredDosage form: System, 52 mgActive substance: plastic IUD with progestogenManufacturer: BAYER OyPrescription required
Alternatives to Levosert Easi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Levosert Easi in Ukraine
Alternative to Levosert Easi in Spain
Online doctors for Levosert Easi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Levosert Easi – subject to medical assessment and local rules.






