JAYDESS 13.5 mg INTRAUTERINE RELEASE SYSTEM


How to use JAYDESS 13.5 mg INTRAUTERINE RELEASE SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Jaydess 13.5mg intrauterine delivery system
levonorgestrel
?This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Jaydess is and what it is used for
- What you need to know before you use Jaydess
- How to use Jaydess
- Possible side effects
- Storing Jaydess
- Contents of the pack and other information
1. What Jaydess is and what it is used for
Jaydess is used for the prevention of pregnancy (contraception) for up to three years.
Jaydess is an intrauterine delivery system (IUS) in the form of a T, which, after being placed inside the uterus, slowly releases a small amount of the hormone levonorgestrel.
Jaydess works by reducing the monthly growth of the uterine lining and thickening the cervical mucus. These actions prevent sperm and egg from coming into contact and thus prevent fertilization of an egg by sperm.
2. What you need to know before you use Jaydess
General considerationsBefore you start using Jaydess, your doctor will ask you some questions about your personal medical history. This leaflet describes several situations in which Jaydess should be removed, or in which the reliability of Jaydess may be reduced. In these situations, you should not have intercourse or should use a condom or other barrier method. Jaydess, like other hormonal contraceptives, does not protect against HIV (AIDS) or any other sexually transmitted disease. Jaydess is not intended for use as an emergency contraceptive (postcoital contraceptive). |
DO NOT use Jaydess
- if you are pregnant (see section "Pregnancy, breastfeeding and fertility")
- if you currently have a pelvic inflammatory disease (PID; infection of the female reproductive organs) or have had this condition several times in the past
- if you have conditions associated with an increased risk of pelvic infections
- if you have an infection of the lower genital tract (an infection of the vagina or cervix)
- if you have had an infection of the uterus after giving birth or after an induced or spontaneous abortion during the last 3 months
- if you currently have abnormal cells on the cervix
- if you have or suspect you have cervical or uterine cancer
- if you have tumors that are sensitive to progestogenic hormones for growth, e.g. breast cancer
- if you have unexplained uterine bleeding
- if you have an anomaly of the cervix or uterus, including fibroids that deform the uterine cavity
- if you have active liver disease or liver tumor
- if you are allergic to levonorgestrel or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Before using Jaydess, inform your doctor if:
- you have diabetes. You usually do not need to change your antidiabetic medication while using Jaydess, but your doctor may need to check it
- you have epilepsy. A seizure (crisis) may occur during insertion or removal
- you have had an ectopic pregnancy (pregnancy outside the uterus) in the past.
Also, talk to your doctor if you have any of the following situations before using Jaydess or if any of them occur for the first time while using this medicine:
- migraine, with visual disturbances or other symptoms that may be signs of a transient cerebral ischemia (temporary obstruction of blood supply to the brain)
- exceptionally severe headache
- jaundice (yellowing of the skin, the whites of the eyes and/or the nails)
- marked increase in blood pressure
- severe arterial diseases such as stroke or heart attack.
The following signs and symptoms could mean that you may have an ectopic pregnancy and you should consult your doctor immediately (see also section "Pregnancy, breastfeeding and fertility"):
- your periods have stopped and then you start having persistent bleeding or pain
- you have severe or persistent pain in the lower abdomen
- you have normal signs of pregnancy, but also have bleeding and feel dizzy
- you have a positive pregnancy test.
Contact your doctor immediately if any of the following situations occur (see also section 4) and remember to inform them that you have Jaydess inserted, especially if it is not the person who inserted it:
- severe pain (like menstrual cramps) or heavy bleeding after insertion or if you experience pain/bleeding that lasts for more than a few weeks. This may be a sign of infection, perforation, or that Jaydess is not in the correct position.
- you no longer feel the threads in your vagina. This may be a sign of expulsion or perforation. You can check by carefully inserting a finger into your vagina and feeling the threads at the end of your vagina, near the opening of the uterus (cervix). Do not pull on the threads, as you may accidentally remove Jaydess. Use a barrier method (such as condoms) until your doctor has checked that the IUS is still in place.
- you or your partner can feel the lower part of Jaydess. Avoid intercourse until your doctor has checked that the IUS is still in place.
- your partner notices the removal threads during intercourse.
- you think you may be pregnant.
- you have persistent abdominal pain, fever, or abnormal vaginal discharge, which may be a sign of infection. Infections should be treated immediately.
- you feel pain or discomfort during intercourse, which may be a sign of infection, ovarian cyst, or that Jaydess is not in the correct position.
- you experience sudden changes in your periods (e.g. if you have scant or no menstrual bleeding and then start experiencing persistent bleeding or pain, or start bleeding heavily), which may be a sign that Jaydess is not in the correct position or has been expelled.
Pads are recommended. If you use tampons or menstrual cups, you should change them carefully to avoid pulling on the Jaydess threads. If you think you may have moved Jaydess from its position (see the list above for possible signs), avoid intercourse or use a barrier method (such as condoms), and contact your doctor.
Psychiatric disorders
Some women who use hormonal contraceptives like Jaydess have reported depression or a depressed mood. Depression can be severe and sometimes may induce suicidal thoughts.
If you experience mood changes and depressive symptoms, contact your doctor for additional medical advice as soon as possible.
Children and adolescents
Jaydess is not indicated for use before the first menstrual bleeding (menarche).
Other medicines and Jaydess
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding and fertility
Pregnancy
Jaydess should not be used during pregnancy.
In some women, menstrual periods may disappear while using Jaydess. Not having periods is not necessarily a sign of pregnancy. If you do not have periods and have other symptoms of pregnancy, you should see your doctor for a check-up and a pregnancy test.
If you have not had a period for six weeks and are concerned, consider taking a pregnancy test. If it is negative, there is no need to take another test unless you have other signs of pregnancy.
If you become pregnant with Jaydess in place, you should see your healthcare provider immediately to have Jaydess removed. Removal may cause an abortion. However, if Jaydess is left in place during pregnancy, not only is the risk of spontaneous abortion higher, but also the risk of preterm birth. If Jaydess cannot be removed, consult your healthcare provider about the benefits and risks of continuing the pregnancy. If the pregnancy continues, you will be closely monitored during the pregnancy and should contact your healthcare provider immediately if you experience stomach cramps, stomach pain, or fever.
Jaydess contains a hormone called levonorgestrel, and isolated cases of effects on the genitals of female babies have been reported if they are exposed to levonorgestrel-releasing intrauterine devices while in the uterus.
If you want to become pregnant, you should contact your doctor to have Jaydess removed.
Ectopic pregnancy (pregnancy outside the uterus)
It is rare to become pregnant while using Jaydess. However, if you do become pregnant while using Jaydess, the risk of the pregnancy being outside the uterus (ectopic pregnancy) is increased. Women who have already had an ectopic pregnancy, tubal surgery, or pelvic infection are at higher risk of this type of pregnancy. An ectopic pregnancy is a serious condition that requires immediate medical attention (see section 2 "Warnings and precautions" for signs and symptoms) and may affect future fertility.
Breastfeeding
You can use Jaydess while breastfeeding. Levonorgestrel (the active ingredient of Jaydess) has been identified in small amounts in the breast milk of breastfeeding women. However, no negative effects have been observed on the growth and development of the baby or on the quantity and quality of breast milk.
Fertility
Your usual level of fertility will return after Jaydess is removed.
Driving and using machines
The influence of Jaydess on the ability to drive and use machines is negligible.
3. How to use Jaydess
- Before inserting Jaydess, you must make sure you are not pregnant.
- Jaydess must be inserted within 7 days from the start of your menstrual period. When inserted during these days, Jaydess works from the insertion and will prevent you from becoming pregnant.
- If Jaydess cannot be inserted within 7 days from the start of your menstrual period, or if your menstrual period is irregular, Jaydess can be inserted on any other day. In this case, you must not have had intercourse without contraceptive methods since your last menstrual period, as well as have a negative pregnancy test before insertion. Additionally, Jaydess may not reliably prevent pregnancy from the moment of insertion. Therefore, you should use a barrier contraceptive method (such as condoms) or abstain from vaginal intercourse for the following 7 days after Jaydess insertion.
- Jaydess is not intended for use as an emergency contraceptive (postcoital contraceptive).
Starting to use Jaydess after giving birth
- Jaydess can be inserted after giving birth once the uterus has returned to its normal size, and not before 6 weeks after delivery (see section 4 "Possible side effects - Perforation").
- See also "Starting to use Jaydess" above for what else you need to know for the time of insertion.
Starting to use Jaydess after an abortion
Jaydess can be inserted immediately after an abortion if the pregnancy lasted less than 3 months, provided there are no genital infections. Jaydess will work from the moment of insertion.
Replacing Jaydess
Jaydess can be replaced at any time during your menstrual cycle with a new Jaydess. Jaydess will work from the moment of insertion.
Switching from another contraceptive method (such as combined hormonal contraceptives, implants)
- Jaydess can be inserted immediately if you can be sure you are not pregnant.
- If more than 7 days have passed since the start of menstrual bleeding, you should abstain from vaginal intercourse or use additional contraceptive protection for the following 7 days.
Insertion of Jaydess
Check-ups by your doctor before insertion may include:
- a cervical smear test (Pap test)
- breast examination
- other tests, e.g. to rule out infections, including sexually transmitted diseases, pregnancy test if necessary. Your doctor will also perform a gynecological examination to determine the position and size of the uterus.
After a gynecological examination:
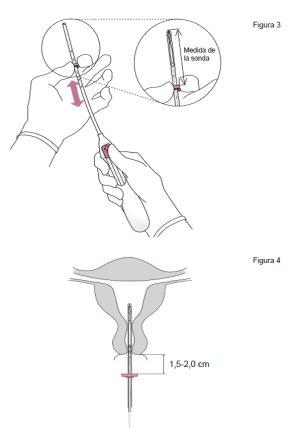
- a device called a speculum is inserted into the vagina and the cervix may be cleaned with an antiseptic solution. Jaydess is then placed in the uterus using a narrow, flexible plastic tube (the insertion tube). Local anesthesia may be applied to the cervix before insertion.
- some women may feel dizzy or faint during or after insertion or removal of Jaydess.
- you may experience some pain and bleeding during or after insertion.
After Jaydess is inserted, your doctor should give you a patient reminder card for follow-up visits. Bring this card to each visit.
Follow-up visit and when to consult your doctor:
You should have Jaydess checked 4-6 weeks after insertion, and then periodically, at least once a year. Your doctor will determine the frequency and type of check-ups that suit your individual case. Bring the patient reminder card that your doctor has given you to each visit. Also, you should contact your doctor if any of the symptoms described in section 2 "Warnings and precautions" occur.
Removal of Jaydess
Jaydess must be removed no later than the end of the third year of use.
Your doctor can easily remove Jaydess at any time, after which pregnancy is possible. Some women may feel dizzy or faint during or after removal of Jaydess. You may experience some pain and bleeding during the removal of Jaydess.
Continuation of contraception after removal
If you do not want to become pregnant, Jaydess should not be removed after the seventh day of the menstrual cycle (menstrual period), unless you use other contraceptive methods (e.g. condoms) for at least 7 days before removal of the IUS.
If you have irregular periods (menstruation) or do not have periods, you should use a barrier contraceptive method for 7 days before removal.
You can also have a new Jaydess inserted immediately after removal, in which case no additional protection is needed. If you do not want to continue with the same contraceptive method, ask your doctor for advice on other reliable contraceptive methods.
4. Possible Adverse Effects
Like all medicines, Jaydess can cause adverse effects, although not all people suffer from them.
Contact your doctor immediately if you notice any of these symptoms:
- Allergic reactions, including rash, hives (urticaria), and angioedema (characterized by sudden swelling of the eyes, mouth, and throat, for example).
Also, see section 2 to know when to contact your doctor immediately.
A list of possible adverse effects is presented below, based on their frequency:
Very Common Adverse Effects:may affect more than 1 in 10 people
- headache
- abdominal/pelvic pain
- acne/oily skin
- menstrual changes, including increased or decreased menstrual bleeding, spotting, infrequent periods, and absence of bleeding (see also the next section on irregular and infrequent bleeding)
- ovarian cyst (see also the next section on ovarian cysts)
- inflammation of the external genital organs and vagina (vulvovaginitis)
Common Adverse Effects:may affect up to 1 in 10 people
- depressed mood/depression
- decreased libido
- migraine
- nausea
- hair loss
- upper genital tract infection
- painful menstruation
- breast pain/discomfort
- expulsion of the device (complete and partial); (see next section on expulsion)
- genital discharge
- weight gain
Uncommon Adverse Effects:may affect up to 1 in 100 people
- dizziness
- excessive body hair
- uterine perforation (see also the next section on perforation)
Description of Selected Possible Adverse Effects:
Irregular or Infrequent Bleeding
It is likely that Jaydess will affect your menstrual cycle. It may change your menstrual periods so that you have spotting (a small amount of bleeding), longer or shorter periods, heavier or lighter bleeding, or complete absence of bleeding.
You may experience bleeding and spotting between menstrual periods, especially during the first 3 to 6 months. Sometimes the bleeding is heavier than usual at first.
In general, it is likely that you will experience a gradual reduction in the amount and number of days of bleeding each month. Some women eventually find that their periods stop completely.
It may be that the monthly thickening of the uterine lining does not occur due to the effect of the hormone, and therefore, there is nothing that can be shed or released in the form of a menstrual period. This does not necessarily mean that you have reached menopause or that you are pregnant. Your own hormone levels usually remain normal.
When the system is removed, the period should return to normal soon.
Pelvic Infection
The Jaydess inserter and Jaydess itself are sterile. Nevertheless, there is a higher risk of pelvic infection (infections of the uterine lining or Fallopian tubes) at the time of insertion and during the first 3 weeks after insertion.
Pelvic infections in IUD users are usually related to the presence of sexually transmitted diseases. The risk of infection increases if you or your partner have multiple sexual partners or if you have had pelvic inflammatory disease (PID) previously.
Pelvic infections must be treated immediately.
Pelvic infections such as PID can have serious consequences and can affect fertility and increase the risk of a future ectopic pregnancy (pregnancy outside the uterus). In extremely rare cases, a severe infection or septicemia (a very serious infection that can be fatal) may occur shortly after insertion.
Jaydess should be removed if you experience recurrent PID or if an infection is severe or does not respond to treatment.
Expulsion
Uterine muscle contractions during menstruation can sometimes push the IUD out of place or expel it. This is more likely to happen if you are overweight at the time of IUD insertion or if you have a history of heavy menstrual periods. If the IUD is displaced, it may not work properly, and the risk of pregnancy increases. If the IUD is expelled, you are no longer protected against pregnancy.
Possible symptoms of an expulsion are pain and abnormal bleeding, but Jaydess can also be expelled without you noticing. Because Jaydess reduces menstrual flow, an increase in flow may be indicative of an expulsion.
It is recommended that you check the threads with your finger, for example, while showering. See also section 2, "Warnings and Precautions" to know how to check if Jaydess is in place. If you present signs that indicate expulsion or are unable to feel the threads, you should use an additional contraceptive method (such as a condom) and consult your healthcare professional.
Perforation
During the insertion of Jaydess, a penetration or perforation of the uterine wall can occur, although the perforation may not be detected until later. If Jaydess is lodged outside the uterine cavity, it is not effective in preventing pregnancy and should be removed as soon as possible. You may need surgery to remove Jaydess. The risk of perforation increases in breastfeeding women and in women who have given birth up to 36 weeks before insertion and may increase in women with a retroverted and fixed uterus. If you suspect that you may have suffered a perforation, seek medical attention quickly and remind them that you have Jaydess inserted, especially if it was not the person who inserted it.
Ovarian Cyst
Since the contraceptive effect of Jaydess is mainly due to its local effect on the uterus, ovulation (release of the egg) usually continues while using this medication. Sometimes an ovarian cyst can form. In most cases, there are no symptoms.
An ovarian cyst may require medical attention or, more rarely, surgery, but it usually disappears on its own.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Jaydess
No special storage conditions are required.
Keep this medication out of sight and reach of children.
Do not open the blister pack. Only your doctor or nurse should do so.
Do not use this medication after the expiration date shown on the packaging and blister pack after "EXP". The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medication in the pharmacy's SIGRE  point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medication. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Jaydess
The active ingredientis levonorgestrel. The intrauterine delivery system contains 13.5 mg of levonorgestrel.
The other componentsare:
- polydimethylsiloxane elastomer
- anhydrous colloidal silica
- polyethylene
- barium sulfate
- black iron oxide (E172)
- silver
Appearance of the Product and Package Contents
Jaydess is a T-shaped intrauterine delivery system. The vertical arm of the T-shaped body has a drug reservoir that contains levonorgestrel. There are two withdrawal strings attached to a loop at the lower end of the vertical arm. Additionally, the vertical arm has a silver ring located near the horizontal arms, which is visible by ultrasound.
Package size:
- 1 x 1 intrauterine delivery system.
- 5 x 1 intrauterine delivery system.
Not all package sizes may be marketed.
Marketing Authorization Holder
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
Bayer Oy
Pansiontie 47
20210 Turku
Finland
This medication is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
- Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Hungary, Iceland, Ireland, Italy, Luxembourg, Malta, Norway, Portugal, Romania, Spain, Sweden: Jaydess
- Estonia, Latvia, Lithuania: Fleree
Date of the last revision of this leaflet: May 2024
Other Sources of Information
You can access detailed and updated information about this medication by scanning the QR code included in the leaflet, packaging, and patient reminder card with your smartphone. You can also access the same information at the following internet address: https://cima.aemps.es/info/77169
---------------------------------------------------------------------------------------------------------------------------
The following information is intended only for healthcare professionals:
INSERTION INSTRUCTIONS
Jaydess 13.5 mg intrauterine delivery system
For insertion by a healthcare professional using an aseptic technique.
Jaydess is supplied within an inserter in a sterile package that should not be opened until it is necessary for insertion. Do not re-sterilize. In this presentation, Jaydess is for single use. Do not use if the blister pack is damaged or opened. Do not insert after the expiration date shown on the packaging and blister pack after "EXP".
Disposal of unused medication or waste material will be carried out in accordance with local regulations.
Jaydess is provided with a patient reminder card within the packaging. Complete the card and give it to the patient after insertion.
Preparation for Insertion
- Examine the patient to rule out contraindications for the insertion of Jaydess (see Summary of Product Characteristics, section 4.3 and section 4.4 under Medical Examination/Consultation).
- Insert a speculum, visualize the cervix, and then carefully clean the cervix and vagina with an appropriate antiseptic solution.
- Assist with a helper if necessary.
- Hold the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to hold the posterior lip of the cervix. Apply gentle traction with the forceps to straighten the cervical canal. The forceps should remain in place and gentle counter-traction should be applied to the cervix throughout the insertion procedure.
- Insert a uterine sound through the cervical canal to the bottom of the uterus to measure the depth and confirm the direction of the uterine cavity and to rule out any possibility of intrauterine anomaly (e.g., septum, submucous fibroids) or the presence of a previously inserted IUD that has not been removed. If difficulties are encountered, consider cervical dilation. If cervical dilation is necessary, consider the use of analgesics and/or a paracervical block.
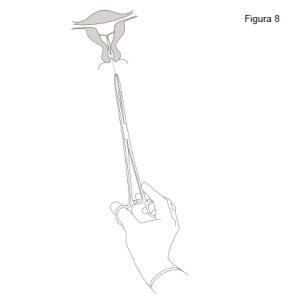
Insertion
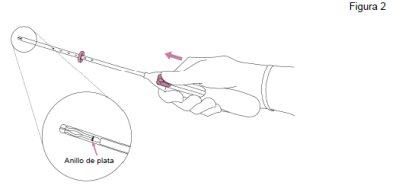
- First, open the sterile package completely. Then, use an aseptic technique and sterile gloves.
|
|
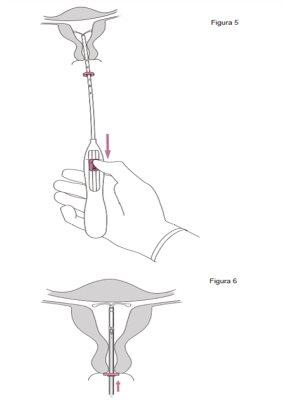
IMPORTANT!Do not pull the slider down because this can release Jaydess prematurely. Once released, Jaydess cannot be reloaded.
|
|
IMPORTANT!Do not force the inserter. Dilate the cervical canal if necessary. |
|
|
|
|
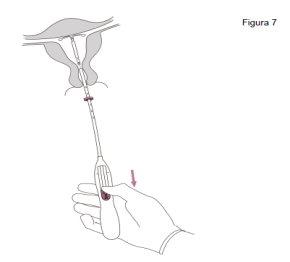
IMPORTANT!If it is suspected that the system is not in the correct position, check its location (e.g., by ultrasound). Remove the system if it is not properly placed within the uterine cavity. A removed system should not be reinserted.
Removal/Replacement
For information on removal/replacement, consult the Jaydess Summary of Product Characteristics.
Jaydess is removed by gently pulling the strings with forceps. A new Jaydess can be inserted immediately after removal. After removal of Jaydess, the system should be examined to ensure that it is intact and that it has been completely removed. |
|
Inclusion at the national level of the QR code that directs to the Summary of Product Characteristics
The Jaydess Summary of Product Characteristics is available at the internet address https://cima.aemps.es/info/77169
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to JAYDESS 13.5 mg INTRAUTERINE RELEASE SYSTEMDosage form: INTRAUTERINE DEVICE, 19.5 mgActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 52 mg / initial release rate of 0.02 mg every 24 hoursActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INTRAUTERINE DEVICE, 0.02 mg/24 hActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for JAYDESS 13.5 mg INTRAUTERINE RELEASE SYSTEM
Discuss questions about JAYDESS 13.5 mg INTRAUTERINE RELEASE SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions