MIRENA 0.02 mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM

How to use MIRENA 0.02 mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Mirena0.02 mg every 24 hours intrauterine delivery system
Levonorgestrel
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the package leaflet
- What is Mirena and what is it used for
- What you need to know before you start using Mirena
- How to use Mirena
- Possible side effects
- Storing Mirena
- Contents of the pack and other information
1. What is Mirena and what is it used for
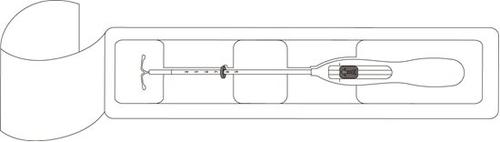
Mirena is a T-shaped intrauterine delivery system (IUS) that, after insertion, releases the hormone levonorgestrel into the uterus. The purpose of the T-shape is to fit the shape of the uterus. The vertical arm of the white T-body contains a drug reservoir that holds levonorgestrel. At the lower end of the vertical arm, there is a loop to which two brown-colored withdrawal threads are attached.
Mirena is used for contraception (prevention of pregnancy) and for heavy menstrual bleeding (idiopathic menorrhagia).
Girls and adolescents
Mirena is not indicated for use before the first menstruation (menarche).
2. What you need to know before you start using Mirena
General notes Before you start using Mirena, your doctor will ask you some questions about your personal and family medical history. This leaflet describes several situations where Mirena should be removed or where the reliability of Mirena may be decreased. In such situations, you should not have intercourse or should take additional non-hormonal contraceptive precautions, for example, use a condom or another barrier method. Do not use the rhythm or temperature method. These methods may not be reliable since Mirena alters the monthly changes in body temperature and cervical mucus. Like other hormonal contraceptives, Mirena does not protect against HIV (AIDS) or any other sexually transmitted disease. |
Do not use Mirena if you have any of the following conditions:
- Pregnancy or suspicion of pregnancy
- Hormone-dependent tumors, e.g., breast cancer
- Current or recurrent pelvic inflammatory disease (infection of the female reproductive organs)
- Cervical infection (cervicitis)
- Lower genital tract infection
- Postpartum uterine infection
- Post-abortal uterine infection in the last 3 months
- Situations associated with increased susceptibility to infections
- Cervical cell abnormalities
- Uterine or cervical cancer, or suspected cancer
- Abnormal uterine bleeding of unknown cause
- Uterine or cervical abnormalities, including fibroids if they deform the uterine cavity
- Acute liver disease or liver tumor
- Allergy to levonorgestrel or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before you start using Mirena.
If you have any of the following conditions or if they occur for the first time while using Mirena, consult a specialist to decide whether you should continue using Mirena or have it removed:
- Migraine, asymmetric vision loss, or other symptoms that may indicate a transient cerebral ischemia (temporary blockage of blood flow to the brain)
- Exceptionally severe headache
- Jaundice (yellowing of the skin, whites of the eyes, and/or nails)
- Marked increase in blood pressure
- Severe arterial diseases, such as stroke or heart attack
- Acute blood clots in the veins.
Mirena should be used with caution in women with congenital heart disease or valvular heart disease with a risk of infective endocarditis.
In diabetic women using Mirena, blood glucose levels should be monitored.
Irrregular bleeding may mask some symptoms and signs of endometrial polyps or cancer, and in these cases, diagnostic tests should be considered.
Examination/medical consultation
The pre-insertion examination may include a cervical smear (Pap test), breast examination, and other tests, for example, for infections, including sexually transmitted diseases, pregnancy test if necessary. A gynecological examination should be performed to determine the position and size of the uterus.
Mirena should not be used as an emergency contraceptive (postcoital contraceptive).
Infections
The insertion tube helps prevent contamination of Mirena by microorganisms during insertion, and the Mirena inserter has been designed to minimize the risk of infection. Nevertheless, users of copper intrauterine devices (IUDs) have a higher risk of pelvic infection immediately after insertion and during the month following insertion. Pelvic infections in IUS users are often related to sexually transmitted diseases. The risk of infection increases if the woman or her partner have multiple sexual partners at the same time. Pelvic infections should be treated promptly. A pelvic infection can impair fertility and increase the risk of a future ectopic pregnancy (pregnancy outside the uterus).
In extremely rare cases, a severe infection or septicemia (a life-threatening infection) may occur shortly after insertion.
Mirena should be removed if recurrent pelvic infections, endometritis, or if an acute infection is severe or does not respond to treatment within a few days.
Consult your doctor immediately if you experience persistent lower abdominal pain, fever, pain during intercourse, or abnormal bleeding.
Expulsion
Uterine muscle contractions during menstruation can sometimes push the IUS out of place or expel it. This is more likely to happen if you are overweight at the time of IUS insertion or if you have a history of heavy menstrual periods. If the IUS is displaced, it may not work properly, and the risk of pregnancy increases. If the IUS is expelled, you are no longer protected against pregnancy.
Possible symptoms of expulsion are pain and abnormal bleeding, but Mirena can also be expelled without you noticing. Since Mirena reduces menstrual flow, an increase in flow may indicate an expulsion.
It is recommended that you check the threads with your finger, for example, while showering. See also section 3 "How to use Mirena - How can I tell if Mirena is in place?". If you experience signs that may indicate expulsion or are unable to feel the threads, you should use an additional contraceptive method (such as condoms) and consult your healthcare provider.
Perforation
Perforation or penetration of the uterine wall can occur, most often during insertion, although it may be detected later. A Mirena that has become embedded outside the uterine cavity is not effective in preventing pregnancy and should be removed as soon as possible. Surgical intervention may be necessary to remove Mirena. The risk of perforation is increased in breastfeeding women and in women who have given birth up to 36 weeks before insertion, and may be increased in women with a fixed, retroverted uterus. If you suspect that you may have experienced a perforation, consult your doctor without delay and remind them that you have Mirena inserted, especially if it is not the person who inserted it.
Possible signs and symptoms of perforation may include:
- severe pain (similar to menstrual cramps) or more severe pain than usual
- heavy bleeding (after insertion)
- prolonged pain or bleeding that persists for more than a few weeks
- sudden change in your periods
- pain during intercourse
- you can no longer locate the Mirena threads (see section 3 "How to use Mirena - How can I tell if Mirena is in place?")
Ectopic pregnancy
It is very unlikely to become pregnant while using Mirena. However, if you do become pregnant while using Mirena, the risk of the fetus developing outside the uterus (ectopic pregnancy) is relatively increased. Approximately 1 in 1,000 women per year who use Mirena correctly will have an ectopic pregnancy. This is lower than women who do not use any contraceptive method (approximately 3 to 5 in 1,000 women per year). A woman who has already had an ectopic pregnancy, tubal surgery, or pelvic infection is at higher risk. An ectopic pregnancy is a serious condition that requires immediate medical attention. The following symptoms may indicate that you have an ectopic pregnancy, in which case you should see your doctor immediately:
- If your periods have stopped but you then experience persistent bleeding or pain
- If you experience diffuse or severe pain in the lower abdomen
- If you experience normal signs of pregnancy but still bleed and feel dizzy.
Dizziness
Some women feel dizzy after the insertion of Mirena. This is a normal physical response. Your doctor will tell you to rest for a moment after the insertion of Mirena.
Ovarian follicles (cells that surround a mature egg in the ovary)
Because the contraceptive effect of Mirena is primarily local, it is usual for women of childbearing age to have ovulatory cycles with follicular rupture. In some cases, the degeneration of the follicle is delayed, and its development may continue. Most of these follicles do not cause symptoms, although some may be accompanied by pelvic pain or pain during intercourse. These enlarged follicles may require medical attention, although they usually disappear on their own.
Psychiatric disorders
Some women who use hormonal contraceptives like Mirena have reported depression or a depressed mood. Depression can be severe and sometimes may induce suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor for further medical advice as soon as possible.
Other medicines and Mirena
The mechanism of action of Mirena is mainly local, and it is not expected that the use of other medicines will increase the risk of pregnancy during the use of Mirena. However, consult your doctor if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy, breastfeeding, and fertility
Pregnancy
Mirena should not be used during pregnancy or suspected pregnancy.
It is very rare for a woman to become pregnant with Mirena in place. But if Mirena is expelled, you will no longer be protected, and you should use another contraceptive method until you see your doctor.
In some women, periods may stop while using Mirena. Not having periods is not necessarily a sign of pregnancy. If you do not have periods and also have other symptoms of pregnancy (e.g., nausea, fatigue, breast tenderness), visit your doctor for a check-up and pregnancy test.
If you become pregnant with Mirena in place, you should see your healthcare provider immediately to have Mirena removed. The removal may cause a miscarriage. However, if Mirena is left in place during pregnancy, not only is the risk of miscarriage increased, but also the risk of preterm birth. If Mirena cannot be removed, consult your healthcare provider about the benefits and risks of continuing the pregnancy. If the pregnancy continues, you will be closely monitored during the pregnancy, and you should contact your doctor immediately if you experience stomach cramps, stomach pain, or fever.
Mirena contains a hormone called levonorgestrel, and there have been isolated reports of effects on the genitals of female babies if they are exposed to levonorgestrel-releasing intrauterine devices while in the uterus.
Breastfeeding
Mirena can be used during breastfeeding. Levonorgestrel has been detected in small amounts in the milk of breastfeeding women (0.1% of the dose is transferred to the child). When Mirena is used, starting six weeks after delivery, it appears to have no negative effects on the growth or development of the child. Progestin-only contraceptives seem to have no effect on either the quantity or quality of breast milk.
Hormonal contraceptives are not recommended as the first-choice contraceptive method during breastfeeding; only non-hormonal methods are considered as such, followed by progestin-only contraceptives like Mirena. The daily dose and blood levels of levonorgestrel are lower than with any other hormonal contraceptive method.
Fertility
After removal of Mirena, women regain their normal fertility.
If you are pregnant, breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
No effects have been observed.
Mirena contains barium sulfate
The T-shaped structure of Mirena contains barium sulfate, which makes it visible on X-ray examination.
3. How to use Mirena
What is the effectiveness of Mirena?
In contraception, Mirena is as effective as the most modern and effective copper IUDs. Studies (clinical trials) showed that about two pregnancies occurred in the first year for every 1,000 women using Mirena.
In the treatment of idiopathic menorrhagia, Mirena produces a marked decrease in menstrual bleeding after three months of use. Some users' periods disappear completely.
When should Mirena be inserted?
Starting to use Mirena
- Before inserting Mirena, you must ensure you are not pregnant.
- Mirena must be inserted within 7 days of the start of menstruation. When inserted during these days, Mirena works from insertion and will prevent pregnancy.
- If Mirena cannot be inserted within 7 days of the start of menstruation or if your menstruation is not regular, then Mirena can be inserted on any other day. In this case, you must not have had unprotected sex since your last menstrual period, and you must have a negative pregnancy test before insertion. Additionally, Mirena may not reliably prevent pregnancy from the moment of insertion. Therefore, you must use a barrier contraceptive method (such as a condom) or abstain from vaginal intercourse for the following 7 days after Mirena insertion.
- Mirena is not indicated for use as an emergency contraceptive (postcoital contraceptive).
Starting to use Mirena after giving birth
- Mirena can be inserted after giving birth, after the uterus has returned to its normal size, and not before 6 weeks after birth (see section 2 "What you need to know before you start using Mirena - Perforation").
- Also, see "Starting to use Mirena" above to know what else you need to know at the time of insertion.
Starting to use Mirena after an abortion
Mirena can be inserted immediately after an abortion if the pregnancy lasted less than 3 months, provided there are no genital infections. Mirena will work from the moment of insertion.
Replacing Mirena
Mirena can be replaced at any time during your menstrual cycle with a new Mirena. Mirena will work from the moment of insertion.
Switching from another contraceptive method (such as combined hormonal contraceptives, implants)
- Mirena can be inserted immediately if you can ensure you are not pregnant.
- If more than 7 days have passed since the start of menstrual bleeding, you must abstain from vaginal intercourse or use additional contraceptive protection for the following 7 days.
How is Mirena inserted?
Mirena must be inserted by a healthcare professional with experience in inserting Mirena.
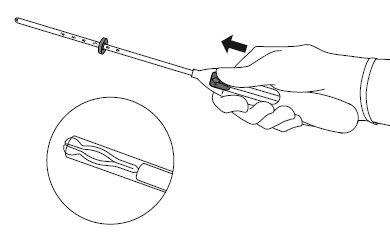
After a gynecological examination, an instrument called a speculum is inserted into the vagina, and the cervix is cleaned with an antiseptic solution. The IUD is inserted into the uterus through a thin, flexible plastic tube (insertor). Local anesthesia may be applied to the cervix before insertion if necessary.
Some women may experience pain and dizziness after insertion. If these do not subside after 30 minutes of rest, it may be due to the IUD not being correctly placed. An examination and removal of the IUD should be performed if necessary.
After Mirena is inserted, your doctor should provide you with a patient reminder card for follow-up visits. Bring this card to each visit.
When should I see my doctor?
You should check your IUD 4-12 weeks after insertion and then regularly, at least once a year. Your doctor will determine the frequency and type of checks that suit your individual case. Bring the patient reminder card that your doctor provided to each visit.
In addition, you should consult your doctor if any of the following situations occur:
- If you no longer feel the removal threads in your vagina
- If you can feel the lower part of the system
- If you think you are pregnant
- If you have prolonged abdominal pain, fever, or abnormal vaginal discharge
- If you or your partner feel pain or discomfort during intercourse
- If you experience sudden changes in your menstruation (e.g., if you have scant or no menstrual bleeding and suddenly start bleeding continuously, or feel pain, or start bleeding heavily)
- If you have other medical problems, such as migraine-type headaches or severe recurring headaches, sudden vision problems, jaundice, or increased blood pressure
- If you have any of the diseases mentioned in section 2 "What you need to know before you start using Mirena".
Remember to tell your doctor that you have Mirena inserted, especially if it's not the person who inserted it.
How long can Mirena be used?
Mirena is effective for 8 years when used for contraception. Are you using Mirena for this reason? If so, your Mirena must be removed or replaced after 8 years at the latest.
Mirena is effective for 5 years for heavy menstrual bleeding (idiopathic menorrhagia). Are you using Mirena for this reason? If so, your Mirena must be removed or replaced when heavy menstrual bleeding recurs or after 8 years at the latest.
If you wish, a new Mirena can be inserted when the old one is removed.
What happens if I want to get pregnant or remove Mirena for any other reason?
The IUD can be easily removed by your doctor at any time, after which pregnancy will be possible. The removal is usually a painless procedure. After Mirena is removed, fertility returns to normal.
Continuation of contraception after removal
If you do not want to get pregnant, Mirena should not be removed after the seventh day of the menstrual cycle (menstrual period), unless contraception is guaranteed with other methods (e.g., condoms) for at least 7 days before removal. If you have irregular periods (menstrual periods) or do not have periods, you should use barrier contraceptive methods from 7 days before removal until your menstruation returns. You can also have a new Mirena inserted immediately after removal; in this case, no additional protection is necessary. If you do not want to continue with the same contraceptive method, ask your doctor for advice on other reliable contraceptive methods.
Can I get pregnant after stopping Mirena?
Yes. After Mirena is removed, it will not interfere with your normal fertility. During the first menstrual cycle after Mirena removal, you can get pregnant.
Can Mirena affect my menstrual periods?
Mirena influences your menstrual cycle. It can change your periods so that you experience spotting (a small amount of blood loss), shorter or longer periods, light or heavy bleeding, or absence of bleeding.
During the first 3-6 months after Mirena insertion, many women experience frequent spotting or light bleeding in addition to their periods. Some women may have heavy or prolonged bleeding during this time. Please inform your doctor, especially if this situation persists.
In general, you will likely have a gradual reduction in the number of days of bleeding and the amount of blood lost each month. Some women, over time, will notice that their period disappears altogether. As the amount of menstrual bleeding usually decreases with Mirena use, most women experience an increase in their hemoglobin blood value.
When the system is removed, the period returns to normal.
Is it abnormal not to have a period?
Not when using Mirena. If you notice you do not have a period while using Mirena, it is due to the effect of the hormone on the uterine lining. The monthly thickening of the lining does not occur. Therefore, there is nothing that can come out in the form of a period. This does not necessarily mean you have reached menopause or are pregnant. Your own hormone levels remain normal.
In fact, not having a period can be a great advantage for women's health.
How can I know if I am pregnant?
Pregnancy is unlikely in women using Mirena, even if they do not have a period.
If you have not had a period for six weeks and are concerned, consider taking a pregnancy test. If it is negative, there is no need to take another test unless you have other signs of pregnancy, such as nausea, fatigue, or breast tenderness.
Can Mirena cause pain or discomfort?
Some women feel pain (like menstrual cramps) during the first weeks after insertion. You should return to your doctor or clinic if you experience severe pain or if the pain persists for more than three weeks after Mirena insertion.
Can Mirena interfere with sexual intercourse?
Neither you nor your partner should feel the IUD during sexual intercourse. If this is not the case, you should avoid sexual intercourse until your doctor checks that the IUD is in the correct position.
How long should I wait after Mirena insertion to have sexual intercourse?
To let your body rest, it is best to wait 24 hours after Mirena insertion before having sexual intercourse. Depending on the time of your menstrual cycle when Mirena is inserted, you may need to use a barrier method (such as a condom) or abstain from sexual intercourse for the first 7 days after insertion (see section 3 "How to use Mirena - When should Mirena be inserted?").
Can I use tampons or menstrual cups?
The use of pads is recommended. If you use tampons or menstrual cups, you must change them carefully to avoid pulling on Mirena's removal threads. If you think you may have moved Mirena from its position (see "When should I see my doctor?" for possible signs), avoid sexual intercourse or use a barrier method (such as condoms), and contact your doctor.
What happens if Mirena comes out?
It is rare but possible for Mirena to come out during your menstruation without you noticing. An unusual increase in the amount of bleeding during your period may mean that Mirena has come out completely through your vagina. It is also possible that part of Mirena has come out of the uterus (you and your partner may notice this during sexual intercourse). If Mirena comes out completely or partially, you will not be protected against pregnancy.
How can I know if Mirena is in place?
You can check if the removal threads are in place. Gently insert a finger into your vagina and feel for the removal threads at the end of your vagina, near the opening of your uterus (cervix).
Do not pull on the removal threadsas you could accidentally pull out Mirena. If you cannot locate the removal threads, this may indicate that an expulsion or perforation has occurred. In this case, you must use a barrier contraceptive method (such as condoms) and consult your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In addition to the possible side effects described in other sections (e.g., section 2 "What you need to know before you start using Mirena"), the following possible side effects are listed by the parts of the body they affect and their frequency:
Very common side effects:may affect more than 1 in 10 people:
Reproductive system and breast disorders
- Uterine or vaginal bleeding, including spotting, infrequent periods (oligomenorrhea), and absence of bleeding (amenorrhea)
- Benign ovarian cysts (see section 2 "Increased ovarian follicles")
Common side effects:may affect up to 1 in 10 people:
Psychiatric disorders
- Depressed mood or depression
- Nervousness
- Decreased libido
Nervous system disorders
- Headache
Vascular disorders
- Dizziness
Gastrointestinal disorders
- Abdominal pain
- Nausea (feeling sick)
Skin and subcutaneous tissue disorders
- Acne
Musculoskeletal, connective tissue, and bone disorders
- Back pain
Reproductive system and breast disorders
- Pelvic pain
- Dysmenorrhea (painful menstruation)
- Vaginal discharge
- Vulvovaginitis (inflammation of the external genital organs or vagina)
- Breast tenderness
- Breast pain
- Expulsion of the intrauterine contraceptive
Investigations
- Weight gain
Uncommon side effects:may affect up to 1 in 100 people:
Nervous system disorders
- Migraine
Gastrointestinal disorders
- Abdominal distension
Skin and subcutaneous tissue disorders
- Hirsutism (excessive hair growth on the body)
- Alopecia (hair loss)
- Pruritus (intense itching)
- Eczema (skin inflammation)
- Chloasma (brownish patches on the skin) or skin hyperpigmentation
Reproductive system and breast disorders
- Uterine perforation
- Pelvic inflammatory disease (infection of the female upper genital tract, above the cervix)
- Endometritis
- Cervicitis/Pap smear normal, class II (inflammation of the cervix)
General disorders and administration site conditions
- Edema (swelling)
Rare side effects:may affect up to 1 in 1,000 people:
Skin and connective tissue disorders
- Rash
- Urticaria (hives)
If you become pregnant while using Mirena, there is a possibility that the pregnancy will develop outside the uterus (see section 2 "Ectopic pregnancy").
Cases of septicemia (a severe, life-threatening infection) have been reported after IUD insertion.
Reporting of side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are possible side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Mirena
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Do not use this medicine after the expiry date stated on the packaging. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Container Contents and Additional Information
Mirena Composition
- The active ingredient is levonorgestrel. The intrauterine release system contains 52 mg of levonorgestrel.
- The other components are polydimethylsiloxane elastomer, anhydrous colloidal silica, polyethylene, barium sulfate, and iron oxide.
Product Appearance and Container Contents
Container contents: a sterile intrauterine release system for intrauterine use.
Marketing Authorization Holder
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
Bayer Oy
Pansiontie 47
20210 Turku
Finland
For any information about this medicinal product, please contact the marketing authorization holder:
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
Spain
IF YOU HAVE ANY FURTHER QUESTIONS, PLEASE ASK YOUR DOCTOR OR PHARMACIST.
Date of Last Revision of this Leaflet:May 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/). Other Sources of Information
You can access detailed and updated information about this medicinal product by scanning the QR code included in the leaflet, packaging, and patient reminder card with your mobile phone (smartphone). You can also access the same information at the following internet address: https://cima.aemps.es/info/63158
-------------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only:
INSERTION INSTRUCTIONS
Mirena 0.02 mg every 24 hours intrauterine release system
It must be inserted by a healthcare professional using an aseptic technique.
Mirena is supplied within an inserter, in a sterile package that should not be opened until it is ready for use. Do not re-sterilize. As supplied, Mirena is for single use only. Do not use if the inner package is damaged or opened. Do not insert after the expiration month and year shown on the label.
To choose the time of insertion, consult the Mirena summary of product characteristics.
Mirena includes a patient reminder card in the package. Complete the patient reminder card and give it to the patient after insertion.
Preparation for Insertion
- Examine the patient to rule out contraindications for the insertion of Mirena and to exclude pregnancy (see Summary of Product Characteristics, section 4.3 and section 4.4 under Examination/medical consultation).
- Insert a speculum, visualize the cervix, and then meticulously clean the cervix and vagina with an appropriate antiseptic solution.
- Assist with a helper if necessary.
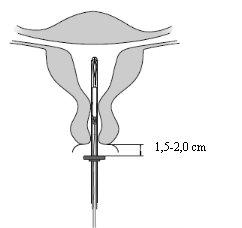
- Hold the anterior lip of the cervix with a tenaculum or other grasping forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to hold the posterior lip of the cervix. Gentle traction can be applied with the forceps to straighten the cervical canal. The forceps should be kept in place and gentle traction should be maintained on the cervix throughout the insertion process.
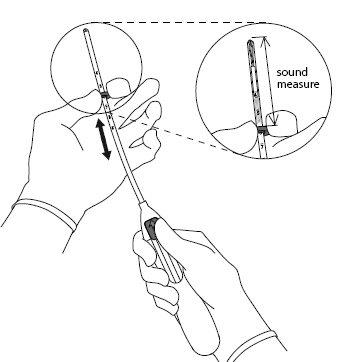
- Introduce a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to exclude any possibility of intrauterine anomaly (e.g., septum, submucous fibroids) or the presence of a previously inserted intrauterine contraceptive that has not been removed. If difficulties are encountered, consider cervical dilation. If cervical dilation is necessary, consider the use of analgesics and/or a paracervical block.
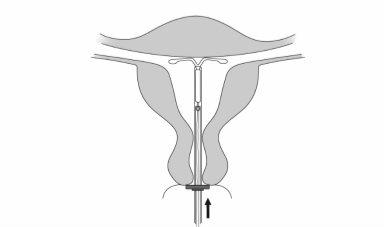
Insertion
| |
| |
|
|
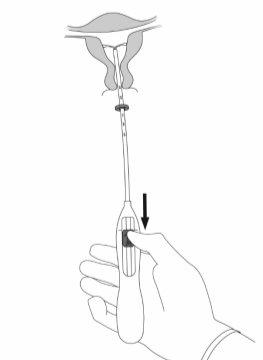
IMPORTANT!Do not pull the slider down because this can release Mirena prematurely. Once released, Mirena cannot be reloaded.
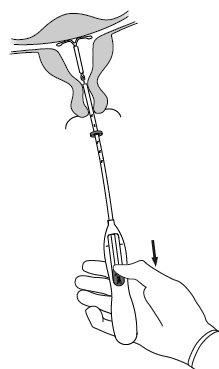
| |
|
|
|
|
IMPORTANT!Do not force the inserter. Dilate the cervical canal if necessary.
| |
|
|
|
|
|
|
IMPORTANT!
If it is suspected that the system is not in the correct position, check its location (e.g., by ultrasound). Remove the system if it is not properly placed within the uterine cavity. A removed system should not be reinserted.
Removal/Reinsertion
For removal/replacement, consult the Mirena summary of product characteristics.
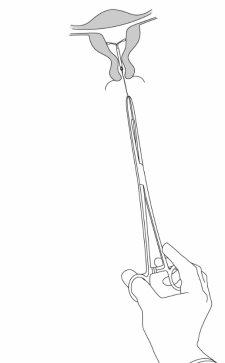
Mirena is removed by gently pulling on the threads with forceps (Figure 8). A new Mirena can be inserted immediately after removal. After removal of Mirena, the system should be examined to ensure that it is intact and that it has been completely removed. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MIRENA 0.02 mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEMDosage form: INTRAUTERINE DEVICE, 13.5 mg levonorgestrelActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 19.5 mgActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 52 mg / initial release rate of 0.02 mg every 24 hoursActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for MIRENA 0.02 mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM
Discuss questions about MIRENA 0.02 mg EVERY 24 HOURS INTRAUTERINE RELEASE SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions