KYLEENA 19.5 mg INTRAUTERINE RELEASE SYSTEM

How to use KYLEENA 19.5 mg INTRAUTERINE RELEASE SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Kyleena and what is it used for
- What you need to know before starting to use Kyleena
- Your doctor/healthcare professional must be sure that this contraceptive is suitable for you.
- As a contraceptive, Kyleena prevents pregnancies. However, no contraceptive prevents all pregnancies.
- The IUS does not protect against HIV infection or any other sexually transmitted disease.
- The IUS is not an emergency contraceptive, such as the morning-after pill.
- How to Use Kyleena
- Possible Side Effects
- Kyleena can be pushed through the uterine wall or go through it. This is called perforation. Perforation usually happens at the time of insertion. Perforation may not always be painful, so you may only notice it later. If it is not in place due to perforation, it will not be effective against pregnancy. In this case, a healthcare professional should remove it as soon as possible. Sometimes, surgery is necessary.
- Perforation can affect up to 1 in 1000 people. The risk of perforation is higher (up to 1 in 100 people) if:
- Storage of Kyleena
- Container Content and Additional Information
Introduction
Package Leaflet: Information for the User
Kyleena 19.5mg Intrauterine Delivery System
levonorgestrel
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor/healthcare professional.
- This medication has been prescribed to you only, and you should not give it to others.
- If you experience side effects, consult your doctor/healthcare professional, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Kyleena and what is it used for
- What you need to know before starting to use Kyleena
- How to use Kyleena
- Possible side effects
- Storage of Kyleena
- Package Contents and Additional Information
1. What is Kyleena and what is it used for
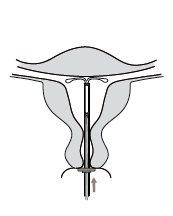
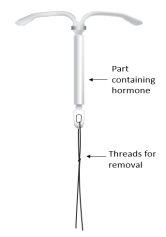
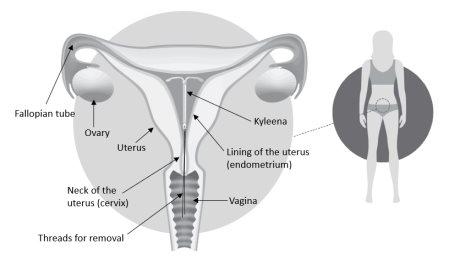
Kyleena is an intrauterine delivery system (IUS) in the shape of a T, also known as a hormonal intrauterine device. It is a contraceptive method that prevents pregnancy for up to 5 years. The IUS contains a hormone called levonorgestrel.
Figure 1: Intrauterine Delivery System


How it works
A doctor/healthcare professional will insert it into your uterus. Once inserted, it continuously releases a small amount of the hormone.
Kyleena prevents sperm and egg from coming into contact and thus prevents pregnancy. It works as follows:
- thickens the cervical mucus, preventing sperm from passing through.
- keeps the uterine lining (endometrium) thin.
Figure 2: Kyleena in the Uterus


2. What you need to know before starting to use Kyleena
Important informationto consider Your doctor/healthcare professional must be sure that this contraceptive is suitable for you.As a contraceptive, Kyleena prevents pregnancies. However, no contraceptive prevents all pregnancies.The IUS does not protect against HIV infection or any other sexually transmitted disease.The IUS is not an emergency contraceptive, such as the morning-after pill. |
Do not use Kyleena if:
- you are pregnant (see the section "Pregnancy, Breastfeeding, and Fertility" below);
- you have a uterine, fallopian tube, or ovarian infection (pelvic inflammatory disease) or have had this condition multiple times in the past;
- you have a disease that makes you more prone to pelvic infections, such as sexually transmitted diseases or diseases that reduce the body's ability to fight infections, such as advanced HIV;
- you have a vaginal or cervical infection;
- you have had a baby or an abortion, spontaneous or not, in the last 3 months and subsequently had a uterine infection;
- your last vaginal cytology results were abnormal;
- you have uterine or cervical cancer, or your doctor/healthcare professional suspects you may have it;
- you have a tumor that needs progestogens to grow, such as breast cancer;
- you have abnormal vaginal bleeding and the cause is unknown;
- you have an abnormally shaped uterus or cervix, such as due to non-cancerous growths in the uterus (uterine fibroids);
- you have liver disease or a liver tumor;
- you are allergic to levonorgestrel or any of the other components of this medication (listed in section 6).
Do not use Kyleena if any of the above applies to you. If you are unsure, consult your doctor/healthcare professional.
Warnings and Precautions
Consult your doctor before starting to use Kyleena if you:
- have diabetes. You usually do not need to change your anti-diabetic medication while using the IUS, but your doctor/healthcare professional may need to check it;
- have epilepsy. A seizure (convulsion) may occur during IUS insertion or removal;
- have had an ectopic pregnancy in the past;
- have migraines that cause vision problems (e.g., sudden loss of vision in one eye) or other types of problems (migraines with aura) or if you have severe headaches of unknown origin;
- have jaundice (yellowing of the skin, nails, and whites of the eyes);
- have high blood pressure;
- have had a stroke or heart attack.
If any of the above applies to you (or you are unsure), consult your doctor/healthcare professional before having Kyleena inserted.
While using Kyleena, consult your doctor/healthcare professional immediately if you:
- have signs of pregnancy or a positive pregnancy test (see the section "Pregnancy, Breastfeeding, and Fertility" below);
- have signs of pregnancy, but also pain, vaginal bleeding, or feel dizzy. This may indicate that you are having an ectopic pregnancy (see section 4, "Ectopic Pregnancy");
- have stomach pain, fever, or unusual vaginal discharge or pain during sex. This may indicate that you have an infection and need to be treated promptly (see section 4, "Pelvic Infection");
- have pain during sex, as you may have a small fluid-filled sac (cyst) on the ovary (see section 4, "Ovarian Cyst");
- have severe pain, heavy bleeding, or no longer feel the IUS threads, as you may have a perforation (see section 4, "Perforation").
Consult your doctor/healthcare professional immediately if you experience any of the above symptoms.
Also, consult your doctor/healthcare professional about Kyleena if you:
- have a migraine or a very severe headache for the first time;
- notice that your skin, nails, and whites of the eyes turn yellow. These are signs of jaundice;
- notice an increase in blood pressure;
- have had a stroke or heart attack.
Your doctor/healthcare professional will decide if it is still safe for you to continue using Kyleena.
Signs that may indicate Kyleena is not in place
Some signs that may indicate that the IUS is not in place are:
- being unable to feel the threads in the vagina (see section 3, "How to check for yourself if Kyleena is in place");
- noticing the lower plastic part (or your partner noticing it) (see section 3, "How to check for yourself if Kyleena is in place");
- having sudden changes in your periods (e.g., you stopped having periods with IUS use and then suddenly start having them again).
These signs may mean that Kyleena has been expelled (see section 4, "Expulsion") or that you have a perforation (see section 4, "Perforation").
If you notice any of these signs that indicate Kyleena is not in place, consult your doctor/healthcare professional immediately. You should not have sex unless you use a condom or diaphragm until your doctor/healthcare professional checks that the IUS is still in place.
It is possible that your partner may feel the threads during sex. This does not mean that the IUS is out of place. However, if your partner is uncomfortable feeling the threads, there are things your doctor/healthcare professional can do to help.
Menstrual Hygiene Products
If you have your period, it is recommended to use pads. If you use tampons or a menstrual cup, you should change them carefully, otherwise, you may accidentally pull on the IUS threads. If you think you may have moved the IUS out of place (see the list above for possible signs), you should not have sex unless you use a condom or diaphragm until you see your doctor/healthcare professional.
Mental Health Problems
Some women who use hormonal contraceptives, including Kyleena, experience depression or depressive mood. See section 4, "Mental Health Problems" for more information.
Children and Adolescents
Girls who have not yet had their period should not use Kyleena.
Other Medications and Kyleena
Tell your doctor/healthcare professional if you are taking, have recently taken, or may need to start taking any other medication.
Pregnancy, Breastfeeding, and Fertility
Pregnancy
Kyleena should not be inserted if you are pregnant.
If you stop having periods while using Kyleena
Some women do not have periods while using it. If you no longer have periods, this is probably due to the IUS. You can find more information about this in section 4, "Irregular or Infrequent Bleeding".
Have you not had a period for 6 weeks? Then you can take a pregnancy test. If the test is negative, you do not need to repeat it.
If you notice symptoms of pregnancy
If you experience symptoms of pregnancy, such as missing periods, nausea, or sore breasts, you should:
- take a pregnancy test;
- contact your doctor/healthcare professional for an examination.
If you become pregnant
If you become pregnant with Kyleena, you should see your doctor/healthcare professional immediately to have it removed.
There is a risk that you may have a miscarriage during removal. However, if you continue to use it throughout pregnancy, you are at a higher risk of:
- having a miscarriage;
- having a premature baby.
Consult your doctor/healthcare professional about the benefits and risks of continuing the pregnancy while using the IUS. Your doctor/healthcare professional will closely monitor you. You should contact your doctor/healthcare professional immediately if you experience:
- cramps;
- stomach pain;
- fever.
Kyleena contains a hormone called levonorgestrel. Ask your doctor/healthcare professional about the effects the hormone may have on the fetus. There have been isolated reports of effects on the genitals of female babies exposed to levonorgestrel IUS while in the uterus.
Ectopic Pregnancy
The risk of becoming pregnant while using Kyleena is very low. However, if you do become pregnant while using it, you are at a higher risk of the fertilized egg being in the fallopian tube or abdominal cavity (ectopic pregnancy) rather than the uterus. An ectopic pregnancy is a serious condition that requires immediate medical attention. After having an ectopic pregnancy, it may be more difficult to become pregnant again. See section 4, "Ectopic Pregnancy".
Breastfeeding
You can use Kyleena while breastfeeding. A small amount of the hormone may pass into breast milk. However, it is unlikely to affect the quantity or quality of breast milk or the growth and development of the infant.
Fertility
If you want to become pregnant, you should contact your doctor/healthcare professional to have Kyleena removed.
Once removed, fertility is not affected.
Driving and Using Machines
Kyleena has no known influence on the ability to drive and use machines.
3. How to Use Kyleena
Starting to Use Kyleena
- Before insertion, you must ensure that you are not pregnant.
- It must be inserted within 7 days of the start of your period. When inserted during these days, it works from the moment of insertion and will prevent pregnancy.
- If it cannot be inserted within 7 days of the start of your period, or if your period is not regular, it can be inserted on any other day. In this case, you must not have had sex without contraception since your last period, and you must have a negative pregnancy test before insertion. Additionally, it may not prevent pregnancy reliably from the moment of insertion. Therefore, you should use a barrier contraceptive method (such as a condom) or abstain from vaginal sex for the next 7 days after insertion.
- Kyleena is not an emergency contraceptive, such as the morning-after pill.
Starting to Use Kyleena After Giving Birth
- It can be inserted after giving birth, once the uterus has returned to its normal size, and not before 6 weeks after delivery (see section 4, "Perforation").
- See also "Starting to Use Kyleena" above for more information on choosing the time of insertion.
Starting to Use Kyleena After an Abortion
It can be inserted immediately after an abortion if the pregnancy was less than 3 months, provided there are no genital infections. The system works from the moment of insertion.
Starting to Use a New Kyleena When the Current One Needs to be Replaced
It can be replaced at any time during your menstrual cycle with a new one. It works from the moment of insertion.
Switching from Another Contraceptive Method (such as Combined Hormonal Contraceptives, Implants)
- Kyleena can be inserted immediately if you can be sure that you are not pregnant.
- If it has been more than 7 days since the start of menstrual bleeding, you should abstain from vaginal sex or use additional contraceptive protection for the next 7 days.
What Happens When Kyleena is Inserted?
Examination Before Insertion
Sometimes, your doctor/healthcare professional may want to perform some type of examination before insertion, including:
- a vaginal cytology
- a breast examination
- other tests (e.g., for sexually transmitted diseases), if necessary.
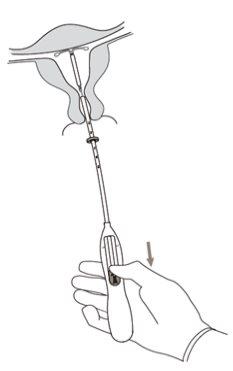
Kyleena Insertion
First, your doctor/healthcare professional will check the size of the uterus and its exact position in the abdomen (pelvic exam).
The doctor/healthcare professional inserts an instrument called a speculum into the vagina and cleans the cervix with an antiseptic liquid. Sometimes, the healthcare professional applies local anesthesia to the cervix and then inserts the IUD into the uterus using a flexible and narrow plastic tube (insertion tube).
Sometimes, insertion can be uncomfortable. Some women experience dizziness or fainting. You may also experience pain and some vaginal bleeding. This is not unusual.
After insertion, the doctor/healthcare professional will give you a card: the patient reminder card. On this card, you can note the date of your next check-up. Bring this card to each visit.
Follow-up After Insertion
A healthcare professional should check the IUD 4-6 weeks after insertion. The doctor/healthcare professional will determine how often you should return for a follow-up examination. You should return for a check-up at least once a year. Bring the patient reminder card to each visit.
How to Check if Kyleena is in Place
You can check by gently inserting a finger into the vagina. You should feel the threads at the top of the vagina, near the cervix. The cervix is the entrance to the uterus. Note: do not pull on the threads, as you could accidentally remove the IUD.
If you do not feel the threads, you should ask your doctor/healthcare professional to check if the IUD is still in place. You should not have sexual intercourse unless you use a condom or diaphragm until you see your doctor/healthcare professional.
If you or your partner notice the lower part of the plastic Kyleena, it is not in place. You should see your doctor/healthcare professional immediately. You should not have sexual intercourse until you see your doctor/healthcare professional, unless you use a condom or diaphragm.
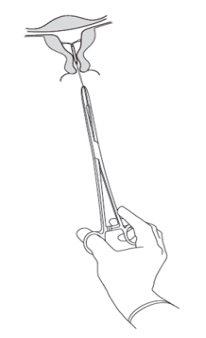
Removal of Kyleena
The IUD has a duration of up to 5 years. It should be removed after 5 years, although it can also be removed before these 5 years. A healthcare professional will remove it. Once removed, you can become pregnant again.
Removal may be somewhat uncomfortable. Some women experience dizziness or fainting during or immediately after removal. You may also experience some pain and vaginal bleeding. This is not unusual.
Continuation of Contraception After Removal
If you do not want to become pregnant after Kyleena is removed, you should know that:
- It is best to remove it within 7 days of the start of your period. If it is removed at another time in your menstrual cycle, you should use a condom or diaphragm during sexual intercourse for 7 days prior to removal.
- If you have irregular or absent periods, you should use a condom or diaphragm during sexual intercourse for 7 days prior to removal. Irregular periods mean that the number of days between menstrual cycles is not always the same.
- You can also have a new IUD inserted immediately after removal, in which case no additional protection is needed. If you do not want to continue with the same contraceptive method, ask your doctor/healthcare professional for advice on other reliable contraceptive methods.
4. Possible Side Effects
Like all medicines, Kyleena can cause side effects, although not everyone gets them.
Serious Side Effects
Serious side effects can occur, so you should contact your doctor/healthcare professional immediately if you experience any of the following:
- Stomach pain, fever, unusual discharge, or abnormal vaginal bleeding or pain during sexual intercourse. This could be an infection of the uterus, fallopian tubes, or ovaries (see the section "Pelvic Infection" below).
- Severe pain similar to menstrual cramps, more pain than expected, or very heavy vaginal bleeding after insertion. Or pain or bleeding that lasts more than a few weeks, changes in menstrual pattern, pain during sexual intercourse, or inability to feel the IUD threads. These can be signs of a perforation (see the section "Perforation" below).
- No period, but then having vaginal bleeding that does not stop, or severe stomach pain that does not go away. These can be signs of an ectopic pregnancy (see the section "Ectopic Pregnancy" below).
- Mood changes and depressive symptoms (see the section "Mental Health Problems" below).
- An allergic reaction, such as a rash, hives, or swelling of the tongue, lips, face, or throat. This type of reaction is very rare.
If you think any of the above applies to you, talk to your doctor/healthcare professional immediately.
Other Side Effects
The following are other side effects you may experience. The most common side effects are listed at the top of this list, and the less common ones are listed at the bottom.
Very Common Side Effects:may affect more than 1 in 10 people
- headache
- abdominal or pelvic pain
- acne or oily skin
- menstrual changes, such as:
- bleeding more or less than usual
- bleeding or spotting outside of your period
- irregular or infrequent periods
- no periods
You can find more information about this in the section "Irregular or Infrequent Periods" below
- ovarian cyst (a small fluid-filled sac in the ovary). You can find more information about this in the section "Ovarian Cyst" below
- vulvovaginitis (inflammation of the vulva and vagina)
Common Side Effects:may affect up to 1 in 10 people
- decreased sexual desire
- migraine
- dizziness
- nausea
- hair loss
- pain during periods
- breast pain or tenderness
- expulsion of the IUD (complete or partial). You can find more information about this in the section "If Kyleena Comes Out" below
- vaginal discharge
- weight gain
Uncommon Side Effects:may affect up to 1 in 100 people
- increased body hair
Description of Selected Possible Side Effects:
Ectopic Pregnancy
Signs of an ectopic pregnancy include:
- no period, but then having persistent vaginal bleeding
- severe or persistent stomach pain
- normal signs of pregnancy, such as nausea or breast tenderness, but also vaginal bleeding and dizziness
- a positive pregnancy test
You should see your doctor/healthcare professional immediately if you experience any of these symptoms.
The risk of becoming pregnant while using Kyleena is very low. However, if you do become pregnant while using it, you are more likely to have an ectopic pregnancy, where the fertilized egg is not in the uterus but in the fallopian tubes or abdominal cavity. About 2 in 1000 women who use it for a year will have an ectopic pregnancy. This type of pregnancy is a serious condition that requires immediate medical attention. You may need to have surgery.
Some women are more likely to have an ectopic pregnancy, including those who:
- have had an ectopic pregnancy before.
- have had surgery on the fallopian tubes.
- have had a pelvic infection.
Irregular or Infrequent Bleeding
It is likely that your period will change due to the IUD. For example:
- You may bleed a little when you are not having your period. This bleeding is called spotting.
- You may have your period less often. In this case, the number of days between menstrual cycles is not always the same.
- You may have shorter or longer periods.
- You may lose more or less blood than usual during your period.
- You may not have your period.
Sometimes, these changes only happen in the first few months after insertion. For example:
- Bleeding when you are not having your period or spotting is more common during the first 3-6 months.
- Some women experience heavier than usual periods at first.
You may gradually lose less blood each month and have a shorter period. Eventually, some women may stop having their period altogether.
Are you not having your period? This is usually normal. Most of the time, this does not mean you are pregnant or menopausal. The reason is that the uterine lining normally thickens each month to prepare for pregnancy and then becomes thin when you have your period. Kyleena reduces the thickening of the uterine lining. This could interrupt your period completely. Your own hormone levels usually remain normal.
When it is removed, you will usually have your period normally again. If you do not, contact your doctor/healthcare professional.
Pelvic Infection
Kyleena is sterile, as is the inserter. However, you can still get a pelvic infection during insertion or in the first 3 weeks after insertion. For example, in the uterine lining, fallopian tubes, or ovaries. This can affect up to 1 in 10 people.
You may experience:
- stomach pain
- fever
- unusual vaginal discharge
- pain during sexual intercourse
You may be more likely to get a pelvic infection if:
- you have a sexually transmitted disease
- you or your partner have multiple sexual partners
- you have had a pelvic infection before
If you have a pelvic infection, it is essential to see your doctor/healthcare professional immediately. Pelvic infection can cause:
- future fertility problems. This may mean having more difficulty getting pregnant
- an ectopic pregnancy if you become pregnant
- a severe or life-threatening infection (septicemia). This is very rare, but it could happen soon after insertion. Having septicemia means you are very sick due to an infection. If left untreated, it can be fatal
Kyleena should be removed if the pelvic infection:
- keeps coming back
- is severe
- does not go away with treatment
If Kyleena Comes Out
Kyleena can be expelled from its position or fall out. This is due to uterine muscle contractions during your period. This can affect up to 1 in 10 people, especially if:
- you are overweight at the time of insertion
- you have had heavy periods before
If the IUD comes out of place, it may not work correctly. You will be more likely to become pregnant. If it comes out, you will no longer be protected against pregnancy.
If the IUD is not in place or comes out, you may feel pain or have vaginal bleeding that is different from usual. You may also not notice that it has come out.
Kyleena usually reduces the amount of blood you lose during your period:
The longer you use it, the less blood you will lose during your period. This means that if you suddenly have heavy periods again, the IUD may have come out. See the section "How to Check if Kyleena is in Place" to learn how to check if the IUD is in place and what to do if you think it is not.
Perforation
Kyleena can be pushed through the uterine wall or go through it. This is called perforation. Perforation usually happens at the time of insertion. Perforation may not always be painful, so you may only notice it later. If it is not in place due to perforation, it will not be effective against pregnancy. In this case, a healthcare professional should remove it as soon as possible. Sometimes, surgery is necessary.
Perforation can affect up to 1 in 1000 people. The risk of perforation is higher (up to 1 in 100 people) if:
- you are breastfeeding when it is inserted
- you have had a baby in the 9 months before insertion
You may have a perforation if:
- you have severe pain similar to menstrual cramps or more pain than expected
- you have heavy vaginal bleeding after insertion
- you have pain or bleeding that lasts more than a few weeks
- you have sudden changes in your menstrual pattern
- you have pain during sexual intercourse
- you can no longer feel the threads
If you think you may have had a perforation, contact a healthcare professional immediately. Remind them that you have an IUD, especially if they did not insert it.
Ovarian Cyst
Sometimes, a small fluid-filled sac can form in the ovary. This sac is called an ovarian cyst.
Signs of an ovarian cyst can be:
- pelvic pain
- pain or discomfort during sexual intercourse
An ovarian cyst usually goes away on its own. However, it may require medical attention or, more rarely, surgery. If you think you may have an ovarian cyst, contact your doctor/healthcare professional.
Mental Health Problems
Some women who use hormonal contraceptives, including Kyleena, experience depression or depressive mood.
Depression can be severe and sometimes lead to suicidal thoughts. If you experience mood changes and depressive symptoms, contact your doctor/healthcare professional as soon as possible. Depression and depressive mood can affect up to 1 in 100 people who use it.
Reporting Side Effects
If you experience any side effects, talk to your doctor/healthcare professional, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Kyleena
No special storage conditions are required.
Keep this medicine out of the sight and reach of children.
Do not open the blister pack (plastic package containing the IUD). Only your doctor or a healthcare professional should do this.
Do not use this medicine after the expiration date stated on the carton and blister pack after EXP. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Container Content and Additional Information
Kyleena Composition
The active ingredientis levonorgestrel. The intrauterine release system contains 19.5 mg of levonorgestrel.
The other componentsare:
- polydimethylsiloxane elastomer
- anhydrous colloidal silica
- polyethylene
- barium sulfate
- polypropylene
- copper phthalocyanine
- silver
Appearance of Kyleena and Container Content
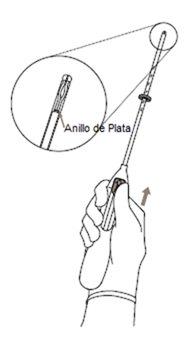
Intrauterine release system (IUS) for uterine use. It has a T-shape and is white. The vertical part is a small reservoir that contains the levonorgestrel hormone. There are two blue threads tied to a loop at the lower end. This allows a healthcare professional to remove the IUS. There is also a silver ring located near the horizontal arms. Your doctor may see this ring during an ultrasound.
Container size:
- 1 x 1 intrauterine release system.
- 5 x 1 intrauterine release system.
Only some package sizes may be marketed.
Marketing Authorization Holder
Bayer Hispania, S.L.
Av. Baix Llobregat 3-5
08970 Sant Joan Despí (Barcelona)
Spain
Manufacturer
Bayer Oy
Pansiontie 47
20210 Turku
Finland
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
- Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Iceland, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden: Kyleena
Date of the last revision of this leaflet:09/2024.
Other sources of information
You can access detailed and updated information about this medicinal product by scanning the QR code included in the leaflet, packaging, and patient reminder card with your mobile phone (smartphone). You can also access the same information at the following internet address: https://cima.aemps.es/info/81418
[National inclusion of the QR code that directs to the technical sheet]
This information is intended only for doctors/healthcare professionals:
INSERTION INSTRUCTIONS
Kyleena 19.5 mg intrauterine release system
levonorgestrel
For insertion by a doctor/healthcare professional using an aseptic technique.
Kyleena is supplied in a sterile container within an inserter that allows for one-handed manipulation. The container should not be opened until it is necessary for insertion. Do not re-sterilize. In this presentation, the IUS is for single use. Do not use if the blister is damaged or opened. Do not insert after the expiration date that appears on the box and on the blister after CAD.
The disposal of unused medicinal products or waste materials will be carried out in accordance with local regulations.
The IUS is provided with a patient reminder card inside the container. Complete the patient reminder card and give it to the patient after insertion.
Preparation for insertion
- Examine the patient to rule out contraindications for insertion (see Technical Sheet, section 4.3 and section 4.4, subsection "Medical examination/consultation").
- Insert a speculum, visualize the cervix, and then carefully clean the cervix and vagina with an appropriate antiseptic solution.
- Assist with a helper if necessary.
- Hold the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to hold the posterior lip of the cervix. Gentle traction can be applied with the forceps to straighten the cervical canal. The forceps should remain in place and gentle counter-traction should be applied to the cervix throughout the insertion procedure.
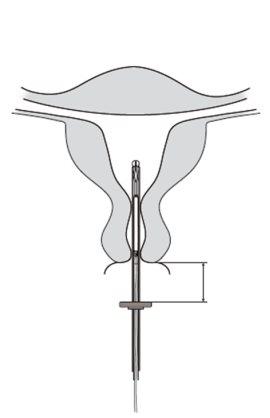
- Insert a uterine sound through the cervical canal to the bottom of the uterus to measure the depth and confirm the direction of the uterine cavity and to rule out any possibility of intrauterine anomaly (e.g., septum, submucous fibroids) or the presence of a previously inserted intrauterine contraceptive that has not been removed. If difficulties are encountered, consider dilating the canal. If cervical dilation is necessary, consider the use of analgesics and/or a paracervical block.
Insertion
- First, completely open the sterile container (Figure 1). Then, use an aseptic technique and sterile gloves.
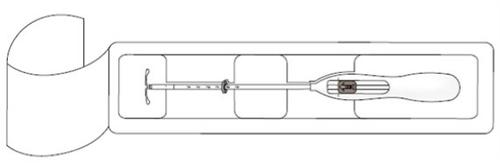







|
| Figure 2 |
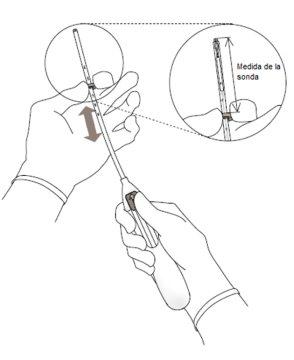
IMPORTANT!Do not pull the slider down, as this may release Kyleena prematurely. Once released, it cannot be reloaded.
|
| Figure 3 |
| 1.5 - 2.0 cm | Figure 4 |
IMPORTANT!Do not force the inserter. Dilate the cervical canal if necessary.
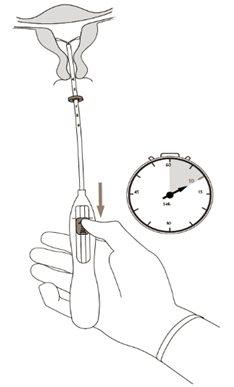
|
| Figure 5 |
|
| Figure 6 |
|
| Figure 7 |
IMPORTANT!If it is suspected that the system is not in the correct position, check its location (e.g., by ultrasound). Remove the system if it is not properly placed within the uterine cavity. A removed system should not be reinserted. |
Removal/Replacement
For removal/replacement, consult the Technical Sheet of Kyleena.
It is removed by pulling on the threads with forceps (Figure 8). A new IUS can be inserted immediately after removal. After removal, the system should be examined to ensure that it is intact and has been completely removed. |
| Figure 8 |
National inclusion of the QR code that directs to the Technical Sheet The Technical Sheet of Kyleena is available at the internet address https://cima.aemps.es/info/81418 |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KYLEENA 19.5 mg INTRAUTERINE RELEASE SYSTEMDosage form: INTRAUTERINE DEVICE, 13.5 mg levonorgestrelActive substance: plastic IUD with progestogenManufacturer: Bayer Hispania S.L.Prescription requiredDosage form: INTRAUTERINE DEVICE, 52 mg / initial release rate of 0.02 mg every 24 hoursActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INTRAUTERINE DEVICE, 0.02 mg/24 hActive substance: plastic IUD with progestogenManufacturer: Gedeon Richter Plc.Prescription required
Online doctors for KYLEENA 19.5 mg INTRAUTERINE RELEASE SYSTEM
Discuss questions about KYLEENA 19.5 mg INTRAUTERINE RELEASE SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions