
How to use Kileena
Leaflet accompanying the packaging: information for the user
Kyleena, 19.5 mg, intrauterine therapeutic system
Levonorgestrel
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if necessary.
- If you have any doubts, you should consult a doctor or nurse.
- This medicine has been prescribed specifically for one person. Do not pass it on to others.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or nurse. See section 4.
Table of contents of the leaflet:
- 1. What Kyleena is and what it is used for
- 2. Important information before using Kyleena
- 3. How to use Kyleena
- 4. Possible side effects
- 5. How to store Kyleena
- 6. Contents of the pack and other information
1. What Kyleena is and what it is used for
Kyleena is an intrauterine therapeutic system in the shape of the letter "T", also known as a hormonal intrauterine device. It prevents pregnancy for up to five years - it is a contraceptive. The Kyleena system contains a hormone called levonorgestrel.
Figure 1: Kyleena hormonal intrauterine device

Hormone-containing element
Removal threads
How Kyleena works
The doctor will place the Kyleena system in the uterus. After its insertion, a small amount of hormone is continuously released.
Kyleena prevents sperm from coming into contact with the egg, and thus prevents pregnancy.
This works as follows:
- it makes the mucus in the cervix thicker. This prevents sperm from entering.
- it keeps the uterine lining (endometrium) thin.
Figure 2: Kyleena system in the uterus
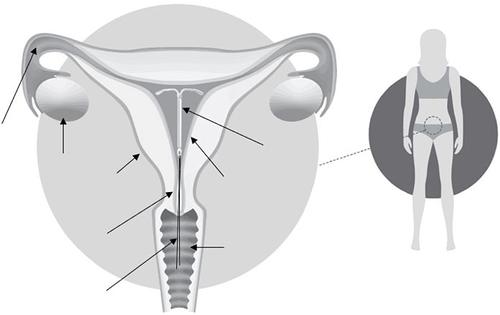
Fallopian tube
Kyleena
Ovary
Uterus
Uterine lining (endometrium)
Cervix
Vagina
Removal threads
2. Important information before using Kyleena
Important information about Kyleena
The doctor must be sure that this contraceptive is suitable for the woman. Therefore, they will first ask a few questions about her health. Only then will it be possible to obtain a prescription.
As a contraceptive, Kyleena prevents pregnancy. However, no contraceptive prevents all pregnancies. Each year, about 2-3 out of 1000 women using the Kyleena system become pregnant.
This system does not protect against HIV or other sexually transmitted diseases.
This system is not an emergency contraceptive, such as the "morning after" pill. Women who have had unprotected sex shortly before its insertion may become pregnant.
When not to use Kyleena:
- if the woman is pregnant (see below "Pregnancy, breastfeeding and fertility"),
- if there is an infection of the uterus, fallopian tubes, or ovaries (pelvic inflammatory disease) or if it has occurred in the woman multiple times in the past,
- if there is a disease that increases the likelihood of pelvic infections. For example: sexually transmitted diseases or diseases that reduce the body's ability to fight infections, such as advanced stages of HIV infection,
- if there is a vaginal or cervical infection,
- if the woman has given birth, had an abortion, or miscarried within the last three months, and then had a uterine infection,
- if the results of the last cytology (cervical screening test) were abnormal,
- if there is cancer of the uterus or cervix or if the doctor suspects cancer,
- if there is a tumor that grows under the influence of progestogens, such as breast cancer,
- if there is vaginal bleeding, and its cause is unknown,
- if the cervix or uterus does not have a normal shape, for example due to non-cancerous changes in the uterus (fibroids),
- if there is liver disease or liver tumor,
- if the woman is allergic to levonorgestrel or any of the other ingredients of this medicine (listed in section 6).
Kyleena should not be used if any of the above situations apply to the woman - in case of doubt, consult a doctor.
Warnings and precautions
Tell your doctor before using Kyleena if:
- the woman has diabetes. Basically, there will be no need to change diabetes medications while using Kyleena, but it may be necessary to check this with a doctor,
- the woman has epilepsy - during the insertion or removal of Kyleena, seizures (epileptic fits) may occur,
- the woman has had an ectopic pregnancy (pregnancy outside the uterus) in the past,
- the woman experiences migraines that cause vision problems - such as sudden loss of vision in one eye - or cause other problems (migraines with aura) or other severe headaches of unknown origin,
- the woman has jaundice (skin, nails, and whites of the eyes turn yellow),
- the woman has high blood pressure,
- the woman has had a stroke or heart attack.
If any of these situations apply to the woman (or if the woman is unsure), she should discuss this with her doctor before inserting Kyleena.
Tell your doctor immediately if, while using Kyleena:
- the woman experiences symptoms of pregnancy, or has a positive pregnancy test result - see below "Pregnancy, breastfeeding and fertility".
- the woman experiences symptoms of pregnancy, but also pain, vaginal bleeding, or dizziness. This may indicate that the woman has become pregnant outside the uterus - see section 4, subsection "Ectopic pregnancy".
- the woman experiences abdominal pain, fever, or unusual vaginal discharge or pain during intercourse - this may indicate an infection and the need for prompt medical attention. See section 4, subsection "Pelvic infection".
- the woman experiences pain during intercourse - a small, fluid-filled sac (cyst) may have formed on the ovary. See section 4, subsection "Ovarian cyst".
- the woman experiences severe pain, very heavy bleeding, or can no longer feel the Kyleena threads in the vagina - this may indicate perforation. See section 4, subsection "Perforation".
If any of the above symptoms occur, the woman should immediately consult her doctor.
The woman should also discuss Kyleena with her doctor if:
- the woman experiences a migraine or a very severe headache for the first time,
- the woman notices that her skin, nails, and whites of the eyes have turned yellow - these are symptoms of jaundice,
- the woman notices an increase in blood pressure,
- the woman has had a stroke or heart attack.
The doctor will decide whether continued use of Kyleena is still safe for the woman.
Pay attention to signs that Kyleena may not be in the correct position
Signs that Kyleena may not be in the correct position include:
- the woman can no longer feel the Kyleena threads with her finger in the vagina - see section 3, subsection "How to check yourself that Kyleena is in the correct position".
- the woman or her partner can feel the lower plastic end of the Kyleena frame - see section 3, subsection "How to check yourself that Kyleena is in the correct position".
- the woman experiences sudden changes in menstrual bleeding. For example: menstrual bleeding stopped after Kyleena was inserted, but then suddenly started again.
These symptoms may indicate that Kyleena has been expelled - see section 4, subsection "Expulsion of Kyleena". Or it may indicate that perforation has occurred - see section 4, subsection "Perforation".
If the woman experiences any of the signs that Kyleena is not in the correct position, she should immediately consult her doctor. The woman should not have sexual intercourse unless a condom or diaphragm is used until the doctor checks that the system is still in the correct position.
The woman's partner may feel the Kyleena threads during intercourse. This does not mean that Kyleena is not in the correct position. However, if the partner feels uncomfortable feeling the threads, there are ways the doctor can help.
Hygiene products during menstruation
During menstruation, it is recommended to use sanitary pads. If tampons or menstrual cups are used, they should be changed carefully. Otherwise, the woman may accidentally pull on the Kyleena threads. If the woman thinks that the system has been displaced (see the list of possible signs above), she should not have sexual intercourse until she visits her doctor, unless a condom or diaphragm is used.
Mental health problems
Some women using hormonal contraceptives, including Kyleena, experience depression or low mood. More information can be found in section 4, subsection "Mental health problems".
Children and adolescents
Girls who have not yet started menstruating should not use Kyleena.
Kyleena and other medicines
The woman should tell her doctor about all medicines she is currently taking or has recently taken, as well as any medicines she plans to take.
Pregnancy, breastfeeding, and fertility Pregnancy
Kyleena should not be inserted during pregnancy.
Stop of menstrual bleeding during use of Kyleena
In some women, menstrual bleeding may not occur during use of Kyleena. If menstrual bleeding has already stopped, it is likely due to Kyleena. More information on this can be found in section 4, subsection "Irregular or rare menstrual bleeding".
Has menstrual bleeding not occurred for 6 weeks? In this case, a pregnancy test can be done.
If the test shows that the woman is not pregnant, there is no need to repeat it.
In case of symptoms of pregnancy
In case of symptoms of pregnancy, such as missed menstrual bleeding, nausea, and tender breasts, the woman should:
- 1. take a pregnancy test,
- 2. contact her doctor for an examination.
In case of pregnancy
If the woman becomes pregnant while using Kyleena, she should immediately contact her doctor. The doctor will decide whether to remove Kyleena.
Removal of Kyleena is associated with a risk of miscarriage. However, leaving the inserted Kyleena system in place during pregnancy may increase the risk of:
- miscarriage,
- premature birth.
The woman should discuss with her doctor the benefits and risks of continuing the pregnancy with the Kyleena system in place. The doctor will closely monitor the woman. The woman should immediately contact her doctor if she experiences:
- abdominal cramps,
- abdominal pain,
- fever.
Kyleena contains a hormone called levonorgestrel. The woman should ask her doctor about the potential effect of the hormone on the developing fetus. Very few reports have been made of the effect of levonorgestrel on the genital organs of female fetuses in the womb.
Ectopic pregnancy
The risk of becoming pregnant while using Kyleena is very low. If the woman does become pregnant while using Kyleena, there is a higher risk that the fertilized egg (embryo) will not implant in the uterus, but in the fallopian tube or abdominal cavity (ectopic pregnancy). This type of pregnancy is a serious condition that requires immediate medical attention. After an ectopic pregnancy, it may be more difficult to become pregnant again. See section 4, subsection "Ectopic pregnancy".
Breastfeeding
Kyleena can be used during breastfeeding. A small amount of hormone passes into breast milk. However, Kyleena does not affect the quality or quantity of breast milk or the growth and development of the breastfed infant.
Fertility
If the woman wants to become pregnant, she should contact her doctor to have Kyleena removed.
Kyleena does not affect fertility after its removal.
Driving and using machines
Kyleena has no known effect on the ability to drive or use machines.
3. How to use Kyleena
Starting to use Kyleena
- Before inserting Kyleena, it must be ensured that the woman is not pregnant.
- Kyleena should be inserted within 7 days of the start of menstrual bleeding. If it is inserted during these days, the system will work immediately and prevent pregnancy.
- If Kyleena cannot be inserted within 7 days of the start of menstrual bleeding, or if menstrual bleeding occurs at an unpredictable time, the system may be inserted on any day. In this case, the woman should not have sexual intercourse without using contraception from the last menstrual bleeding until the insertion of Kyleena, and she should have a negative pregnancy test result. Additionally, Kyleena may not prevent pregnancy immediately in a reliable manner. Therefore, the woman should use a barrier method of contraception (e.g., condoms) or abstain from sexual intercourse for the first 7 days after insertion.
Kyleena is not an emergency contraceptive, such as the "morning after" pill.
Starting to use Kyleena after childbirth
- Kyleena can be inserted after childbirth, when the uterus has returned to its normal size, but not earlier than 6 weeks after childbirth (see section 4, subsection "Perforation").
- See also "Starting to use Kyleena" above to learn more about the timing of insertion.
Starting to use Kyleena after a miscarriage
Kyleena can be inserted immediately after a miscarriage, if the pregnancy lasted less than 3 months and there are no genital tract infections, and it will work immediately.
Replacing Kyleena when the current system needs to be replaced
The system can be replaced with a new one at any time during the menstrual cycle, and it will work immediately.
Switching from another contraceptive method (e.g., combined hormonal contraceptives, implant)
- Kyleena can be inserted immediately if it is justified that the woman is not pregnant.
- If more than 7 days have passed since the start of menstrual bleeding, the woman should abstain from sexual intercourse or use additional contraceptive protection for the next 7 days.
What happens during the insertion of Kyleena? Examination before insertion
Sometimes, before inserting Kyleena, the doctor may perform certain examinations, such as:
- a cervical smear (cytology),
- a breast examination,
- other examinations, if necessary, such as tests for sexually transmitted diseases.
Insertion of Kyleena
First, the doctor will examine the size of the uterus and its position in the abdominal cavity (pelvic examination).
The doctor will insert a device (speculum) into the vagina and clean the cervix with an antiseptic solution. Sometimes, the doctor may use local anesthesia on the cervix. Then, the doctor will insert Kyleena into the uterus through a thin, flexible, plastic tube (applicator).
Sometimes, the insertion of Kyleena may cause discomfort. Some women may feel dizzy or faint. There may also be pain or slight bleeding from the vagina. This is not unusual.
After the insertion is complete, the doctor will give the woman a patient reminder card. This card can be used to record the dates of subsequent Kyleena checks. The woman should bring this card to every visit.
Follow-up after insertion
Kyleena should be checked by a doctor 4-6 weeks after its insertion.
The doctor will decide how often subsequent check-ups should be. Kyleena should be checked at least once a year during a visit. The woman should bring the patient reminder card to every visit.
How to check yourself that Kyleena is in the correct position
This can be checked by gently inserting a finger into the vagina. The threads should be felt at the end of the vagina, near the cervix. The cervix is the entrance to the uterus. Note: Do not pull on the threads, as this can cause the Kyleena system to be accidentally removed.
If the threads are not felt, the doctor must check that Kyleena is still in the correct position. The woman should not have sexual intercourse unless a condom or diaphragm is used until she visits her doctor.
If the woman or her partner can feel the lower plastic end of the Kyleena frame- Kyleena is not in the correct position. The woman should immediately contact her doctor. The woman should not have sexual intercourse until she visits her doctor, unless a condom or diaphragm is used.
Removal of Kyleena
Kyleena works for up to 5 years. It should be removed after 5 years, but it can also be removed at any time before 5 years. The doctor will remove the system. After its removal, the woman can become pregnant.
Removal of Kyleena may be uncomfortable. Some women may feel dizzy or faint during or immediately after removal. There may also be slight pain or bleeding from the vagina. This is not unusual.
Continuing contraception after removal
If the woman does not want to become pregnant after Kyleena is removed, she should know that:
- it is best to remove Kyleena within 7 days of the start of the menstrual cycle. If it is removed at a different time than menstruation, a condom or diaphragm should be used during sexual intercourse for 7 days before removal.
- if the woman has irregular menstrual bleeding or does not menstruate at all, a condom or diaphragm should be used during sexual intercourse for 7 days before removal. Irregular menstrual bleeding means that the number of days between menstrual cycles is not always the same.
- a new Kyleena system can also be inserted immediately after removal. In this case, no additional protection is necessary. If the woman does not want to continue using the same method, she should ask her doctor for advice on other proven contraceptive methods.
4. Possible side effects
Like all medicines, Kyleena can cause side effects, although not everybody gets them.
Severe side effects
There are certain severe side effects, which means that if they occur, the woman should immediately contact her doctor:
- abdominal pain, fever, unusual vaginal discharge, or abnormal vaginal bleeding or pain during sexual intercourse - this may be an infection of the uterus, fallopian tubes, or ovaries - see below "Pelvic infections".
- severe pain, like menstrual cramps, or pain greater than expected, or very heavy vaginal bleeding after insertion. If there is pain or bleeding that lasts longer than a few weeks, sudden changes in the menstrual cycle, pain during sexual intercourse, or the Kyleena threads are no longer felt, these may be symptoms of perforation - see below "Perforation".
- menstrual bleeding has stopped, but there is vaginal bleeding that does not stop, or abdominal pain that is severe or does not stop - these may be symptoms of an ectopic pregnancy - see below "Ectopic pregnancy".
- there are changes in mood and symptoms of depression - see below "Mental health problems".
- an allergic reaction - such as a skin rash, hives, or swelling of the tongue, lips, face, or throat. This type of reaction is very rare.
If the woman thinks that any of the above points apply to her, she should immediately contact her doctor.
Other side effects
The following are other side effects that may occur. The most common side effects are at the top of the list, and the rarest are at the bottom.
Very common side effects:occur in more than 1 in 10 people
- headache
- abdominal pain or pelvic pain
- acne or oily skin
- changes in menstrual bleeding, such as: heavier or lighter bleeding than usual, bleeding or spotting outside of menstruation, irregular or rare menstrual bleeding, or absence of menstruation. More information on this can be found in the section "Irregular or rare menstrual bleeding" below.
- a small, fluid-filled sac (cyst) on the ovary. More information on this can be found in the section "Ovarian cyst" below.
- vaginal inflammation
Common side effects:occur in less than 1 in 10 people
- decreased libido
- migraine
- dizziness
- nausea
- hair loss
- menstrual cramps
- breast pain or tenderness
- spontaneous expulsion of Kyleena (complete or partial). More information on this can be found in the section "Expulsion of Kyleena" below.
- vaginal discharge
- weight gain
Uncommon side effects:occur in less than 1 in 100 people
- excessive hair growth on the body
Description of selected possible side effects:
Ectopic pregnancy
Symptoms of an ectopic pregnancy include:
- menstrual bleeding has stopped, but then vaginal bleeding starts again and does not stop,
- severe abdominal pain that does not stop,
- normal symptoms of pregnancy, such as nausea or tender breasts, but also vaginal bleeding and dizziness,
- a positive pregnancy test result.
The woman should immediately contact her doctor if she experiences any of these symptoms.
The risk of becoming pregnant while using Kyleena is very low. If the woman does become pregnant while using Kyleena, there is a higher risk that the fertilized egg (embryo) will not implant in the uterus, but in the fallopian tube or abdominal cavity (ectopic pregnancy). This type of pregnancy is a serious condition that requires immediate medical attention. About 2 out of 1000 women using Kyleena for a year will have an ectopic pregnancy. This type of pregnancy can require surgery.
Some women are more likely to have an ectopic pregnancy. These include women:
- who have had an ectopic pregnancy before,
- who have had fallopian tube surgery,
- who have had a pelvic infection.
Irregular or rare menstrual bleeding
It is likely that menstrual bleeding will change due to the use of Kyleena. For example:
- there may be slight bleeding outside of menstruation. This bleeding is called spotting.
- menstrual bleeding may become less regular. In this case, the number of days between menstrual cycles is not always the same.
- menstrual bleeding may be shorter or longer.
- bleeding may be less or more intense than usual during menstruation.
- menstrual bleeding may stop altogether.
Sometimes, these changes occur only in the first few months after insertion. For example:
- bleeding outside of menstruation or spotting occurs most often within the first 3 to 6 months.
- in some women, menstrual bleeding is initially more intense than usual.
Gradually, the woman may experience less intense menstrual bleeding, and menstrual bleeding may become shorter. Eventually, some women may stop menstruating altogether.
No menstrual bleeding? This is usually normal. In most cases, it does not mean that the woman is pregnant or going through menopause. The reason is that normally, the uterine lining thickens every month to prepare for pregnancy, and then becomes thinner again due to menstruation. Kyleena reduces the thickening of the uterine lining. This can cause menstrual bleeding to stop.
Hormone levels usually remain normal.
After removal of Kyleena, normal menstrual bleeding usually returns. If it does not, the woman should contact her doctor.
Pelvic infection
Kyleena does not contain bacteria, viruses, or fungi (it is sterile). This also applies to the applicator. However, a pelvic infection can still occur during the insertion of Kyleena or within the first 3 weeks after its insertion. For example, in the uterine lining, fallopian tubes, or ovaries. This can affect less than 1 in 10 women.
The following may occur:
- abdominal pain,
- fever,
- unusual vaginal discharge,
- pain during sexual intercourse.
The risk of pelvic infection is higher if:
- the woman has a sexually transmitted disease,
- the woman or her partner has multiple sexual partners,
- the woman has had a pelvic infection in the past.
In case of a pelvic infection, it is essential to immediately contact a doctor. A pelvic infection can cause:
- future fertility problems. This may mean that it will be more difficult to become pregnant,
- an ectopic pregnancy (a pregnancy outside the uterus) if the woman becomes pregnant,
- a severe infection or blood poisoning. This occurs very rarely and soon after the insertion of Kyleena. Blood poisoning is a very serious disease caused by an infection. If left untreated, blood poisoning can be fatal.
Kyleena must be removed if a pelvic infection:
- occurs repeatedly,
- is very severe,
- does not resolve with treatment.
Expulsion of Kyleena
Kyleena may be expelled or fall out. This is caused by uterine contractions during menstruation. This can occur in less than 1 in 10 women, especially if:
- the woman is overweight at the time of Kyleena insertion,
- the woman has had heavy menstrual bleeding in the past.
If Kyleena is not in the correct position, it may not work properly. The risk of becoming pregnant is higher in this case. If it is expelled, it will no longer prevent pregnancy.
If Kyleena is not in the correct position or has been expelled, the woman may experience pain or bleeding from the vagina that is different from normal. It is also possible that Kyleena has been expelled without the woman noticing.
Kyleena usually reduces the amount of blood lost during menstruation.
The longer the woman uses it, the less blood she loses during menstruation. This means that if the woman suddenly starts to lose more blood during menstruation again, Kyleena may have been expelled.
In section 3, "How to check yourself that Kyleena is in the correct position", you can find information on how to check if it is in the correct position and what to do if you suspect that it is no longer in the correct position.
Perforation
It may happen that Kyleena is pushed into the uterine wall or through the uterine wall. This is called perforation. Perforation usually occurs during the insertion of Kyleena.
Perforation does not always cause pain, so it may only be noticed later. If Kyleena is no longer in the correct position due to perforation, it will not prevent pregnancy. The doctor must then remove it as soon as possible. Sometimes, surgery is necessary.
Perforation occurs in less than 1 in 1000 women. The risk of perforation is higher (in less than 1 in 100 women) if:
- the woman is breastfeeding at the time of Kyleena insertion,
- the woman has given birth within the last 9 months before Kyleena insertion.
Perforation may have occurred if:
- there is severe pain, like menstrual cramps, or pain greater than expected,
- there is very heavy vaginal bleeding after insertion,
- there is pain or bleeding that lasts longer than a few weeks,
- there are sudden changes in the menstrual cycle,
- there is pain during sexual intercourse,
- the Kyleena threads are no longer felt.
If the woman thinks that perforation may have occurred, she should immediately contact her doctor.
The woman should tell her doctor about Kyleena, especially if it is not the same doctor who inserted the system.
Ovarian cyst
Sometimes, during the use of Kyleena, a small, fluid-filled sac (cyst) forms on the ovary.
Symptoms of an ovarian cyst may include:
- pelvic pain,
- pain or discomfort during sexual intercourse.
The ovarian cyst usually resolves on its own. However, it may require medical attention. In rare cases, surgery may be necessary. If the woman thinks that she may have an ovarian cyst, she should contact her doctor.
Mental health problems
Some women using hormonal contraceptives, including Kyleena, experience depression or low mood.
Depression can be severe and sometimes lead to suicidal thoughts. If there are changes in mood and symptoms of depression, the woman should contact her doctor as soon as possible. Depression and low mood can occur in less than 1 in 100 women using Kyleena.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, the woman should consult her doctor or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Kyleena
There are no special precautions for storing the medicine.
The medicine should be stored out of sight and reach of children.
Do not open the blister pack (the plastic container in which Kyleena is stored). Only a doctor or nurse can do this.
Kyleena should not be inserted after the expiration date stated on the packaging and blister pack after: Expiration date (EXP). The expiration date refers to the last day of the specified month.
6. Contents of the pack and other information
What Kyleena contains
The active substance of the medicine is levonorgestrel. The intrauterine therapeutic system contains 19.5 mg of levonorgestrel.
Other ingredientsare:
- polydimethylsiloxane elastomer
- silica, colloidal anhydrous
- polyethylene
- barium sulfate
- polypropylene
- copper phthalocyanine
- silver
What Kyleena looks like and what the pack contains
Kyleena is a hormonal intrauterine device (intrauterine therapeutic system). It is in the shape of the letter "T" and is white. On the vertical part, there is a small container with the hormone levonorgestrel. Two blue threads are attached to the loop at the bottom of the system, which can be used by the doctor to remove Kyleena if necessary. A silver ring near the horizontal arms of the Kyleena system is visible to the doctor during an ultrasound examination.
Pack size:
- 1x1 intrauterine therapeutic system.
- 5x1 intrauterine therapeutic system.
Not all pack sizes may be marketed.
Marketing authorization holder
Bayer AG
Kaiser-Wilhelm-Allee 1
51373 Leverkusen,
Germany
Manufacturer
Bayer OY
Pansiontie 47
20210 Turku
Finland
To obtain more detailed information on this medicine, please contact the local representative of the marketing authorization holder:
Bayer Sp. z o.o.
Al. Jerozolimskie 158
02-326 Warsaw
tel. (0-22) 572 35 00
This medicine is authorized in the Member States of the European Economic Area under the following names:
- Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, Germany, Iceland, Ireland, Italy, Latvia, Lithuania, Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden: Kyleena
Date of last revision of the leaflet:September 2024
Other sources of information
Detailed and up-to-date information on this medicine can be obtained by scanning the QR code attached to the patient leaflet, packaging, and patient reminder card using a smartphone. The same information is also available at the following URL: www.pi.bayer.com/kyleena/pl .
[QR code for the patient leaflet, to be added at the national level]
--------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
INSTRUCTIONS FOR INSERTION
Kyleena, 19.5 mg, intrauterine therapeutic system
Levonorgestrel
To be inserted by a doctor, using aseptic techniques.
The Kyleena system is supplied in a sterile package with an integrated applicator, which allows for one-handed insertion. The package should not be opened until the insertion procedure is about to begin. Do not resterilize. In its provided form, the Kyleena system is intended for single use only. Do not use if the blister is damaged or opened. Do not insert after the expiration date printed on the carton and blister pack: Expiration Date (EXP).
Any unused medicinal product or waste material should be disposed of in accordance with local regulations.
The Kyleena system is supplied with a patient reminder card in the outer packaging.
The patient reminder card should be completed and given to the woman after the system has been inserted.
Preparation for Insertion
- Examine the woman to rule out contraindications for the insertion of the Kyleena system (see Summary of Product Characteristics, section 4.3, and "Examinations/medical consultations" in section 4.4).
- Insert a speculum, visualize the cervix, and then thoroughly swab the cervix and vagina with an appropriate antiseptic solution.
- If necessary, use an assistant for support.
- Grasp the cervix with surgical forceps or other clamps at the anterior lip to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip. Gentle traction on the forceps may straighten the cervical canal. The forceps should remain in this position, and gentle traction on the cervix should be maintained during the insertion procedure.
- Insert a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to rule out any abnormalities within the uterus (e.g., septa, submucous fibroids) or previously inserted intrauterine contraceptives that have not been removed. If difficulty is encountered, consider dilating the cervix. If cervical dilation is necessary, consider using analgesics and/or a paracervical block.
Insertion
- 1. First, completely open the sterile package (Figure 1). Then, apply aseptic technique and wear sterile gloves.
Figure 1
Kyleena
Alignment mark
Handle with threads
inside
Applicator tube
with plunger and scale
Slider
Limiter
- 2. Move the slider forwardin the direction indicated by the arrow until it stops, to load the Kyleena system into the insertion tube (Figure 2).
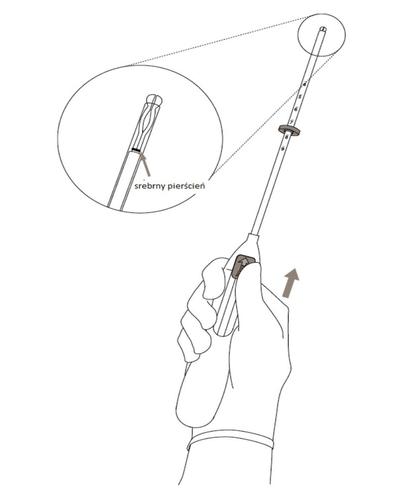
Figure 2
IMPORTANT!Do not move the slider downward, as this may cause premature release of the Kyleena system. Once released, the Kyleena system cannot be reloaded.
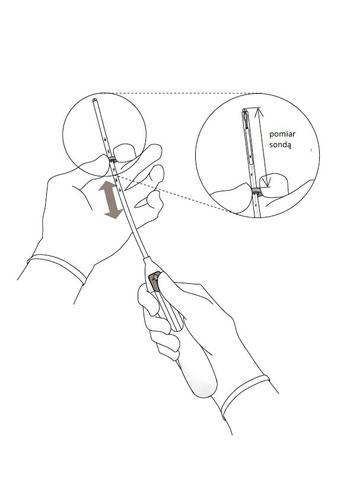
- 3. Holding the slider in its most forward position, set the upperedge of the limiter to correspond to the measured uterine depth (Figure 3). Figure 3
- 4. Holding the slider in its most forwardposition, insert the applicator tube through the cervix until the limiter is about 1.5-2.0 cm from the cervix (Figure 4).
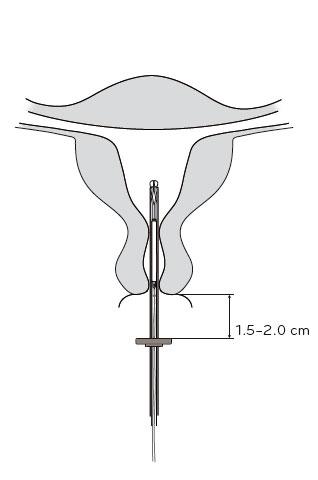
Figure 4
IMPORTANT!Do not force the applicator. If necessary, dilate the cervical canal.
- 5. Holding the applicator steady, move the slider to the alignment markto open the horizontal arms of the Kyleena system (Figure 5). Wait 5-10 seconds to allow for complete opening of the horizontal arms. Figure 5
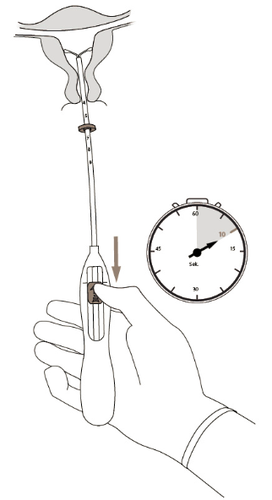
- 6. Gently advance the applicator toward the fundus of the uterus until the limiter touches the cervix. The Kyleena system is then in the correct position at the fundus (Figure 6).
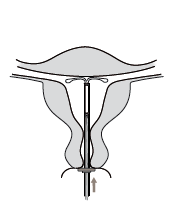
Figure 6
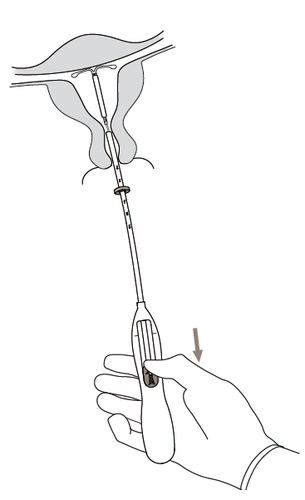
- 7. Holding the applicator in place, release the Kyleena system by moving the slider completely downward(Figure 7). Holding the slider completely down, gently remove the applicator by withdrawing it.
Cutting the Threads
leaving
2-3 cm, which will be
visible outside the
cervix.
Figure 7
IMPORTANT!If it is suspected that the system is not in the correct position, its location should be checked (e.g., by ultrasound). Remove the system if it is not correctly placed in the uterine cavity. A removed system should not be reinserted.
Removal/Replacement of the System
To remove/replace the system, refer to the Kyleena Summary of Product Characteristics.
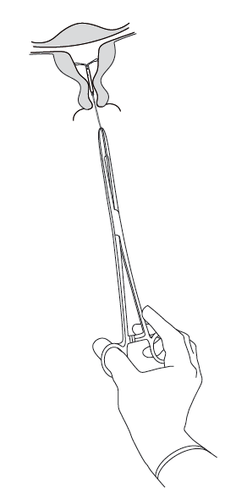
Kyleena system
is removed by
gently pulling on
the threads with
forceps (Figure 8).
A new Kyleena
system can be
inserted immediately
after removal of
the previous one.
After removal of the
Kyleena system, it
should be evaluated
to ensure that it has
not been damaged and
has been completely
removed.
Figure 8
[QR code for the Summary of Product Characteristics, to be added at the national level]
Kyleena Summary of Product Characteristics online at www.pi.bayer.com/kyleena/pl.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBAYER Oy
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KileenaDosage form: System, 52 mg (20 mcg/24h)Active substance: plastic IUD with progestogenPrescription requiredDosage form: System, 52 mg (20 mcg/24 h)Active substance: plastic IUD with progestogenPrescription requiredDosage form: System, 52 mgActive substance: plastic IUD with progestogenManufacturer: BAYER OyPrescription required
Alternatives to Kileena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Kileena in Ukraine
Alternative to Kileena in Spain
Online doctors for Kileena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Kileena – subject to medical assessment and local rules.






