How to use Mirena
Leaflet accompanying the packaging: information for the user
Warning! Keep the leaflet! Information on the immediate packaging in a foreign language.
Mirena
52 mg, 20 micrograms/24 hours, intrauterine therapeutic system
Levonorgestrel
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If you experience any side effects, including any not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What Mirena is and what it is used for
- 2. Important information before using Mirena
- 3. How to use Mirena
- 4. Possible side effects
- 5. How to store Mirena
- 6. Contents of the packaging and other information
1. What Mirena is and what it is used for
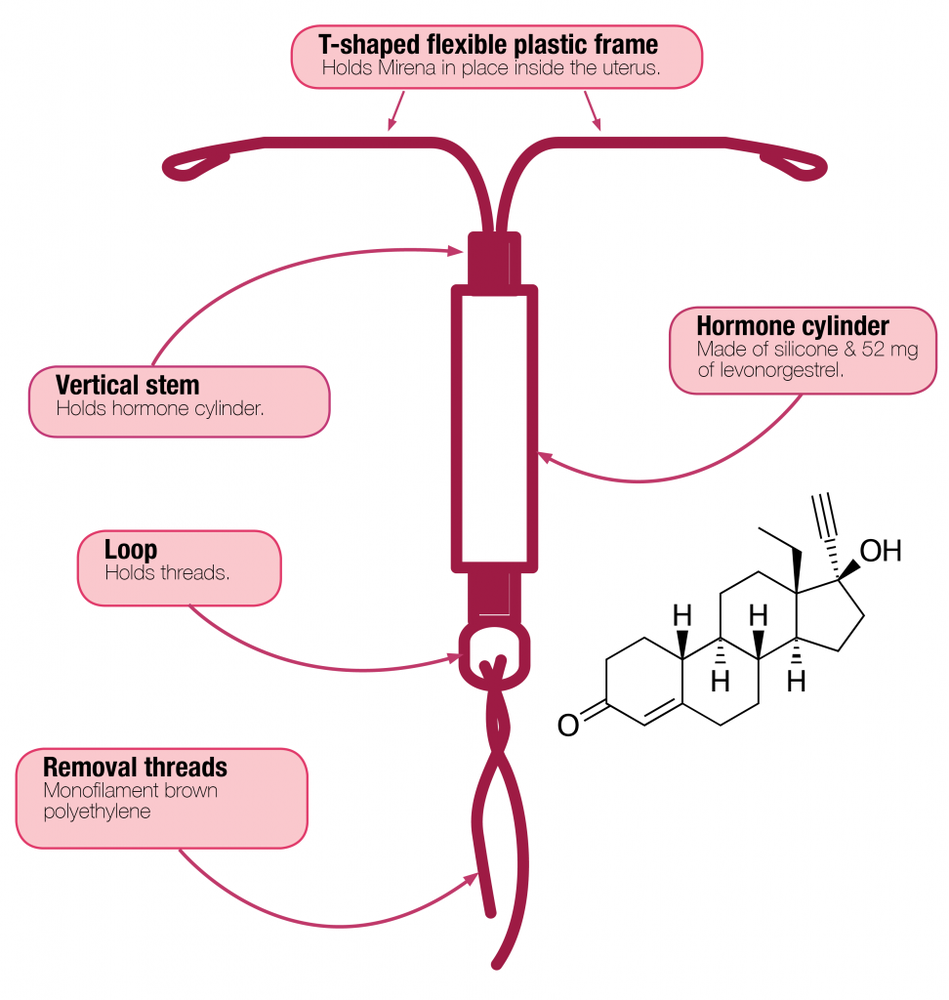
Mirena is a T-shaped intrauterine therapeutic system that releases the hormone levonorgestrel into the uterus after insertion. The T-shape ensures that the system fits into the uterus. The white system has a reservoir containing levonorgestrel in the vertical part. Two brown threads are attached to the loop at the bottom of the system, which are used to remove the system from the uterus.
Mirena is used to prevent pregnancy (contraceptive effect) and to treat heavy menstrual bleeding (of unknown origin).
Children and adolescents
Mirena is not indicated for use before the first menstrual period.
2. Important information before using Mirena
General notes
Before using the Mirena system, the doctor will ask the patient a few questions about her health and the health of her close relatives.
This leaflet describes several situations in which the Mirena system should be removed or when its effectiveness may be reduced. In such cases, you should either abstain from sexual intercourse or use additional non-hormonal contraceptive methods, such as a condom or another mechanical method. You should not use the calendar method or temperature measurement. They may be ineffective because Mirena affects the monthly changes in body temperature and cervical mucus.
Mirena, like other hormonal contraceptives, does not protect against HIV (AIDS) or other sexually transmitted diseases.
Mirena should not be inserted if any of the following conditions are present:
- if the patient is allergic to levonorgestrel or any of the other ingredients of this medicine (listed in section 6)
- pregnancy or suspected pregnancy
- tumors that are dependent on progesterone, such as breast cancer
- existing or recurrent inflammatory conditions of the pelvic organs (infections of the female reproductive organs)
- cervicitis
- lower genital tract infections
- postpartum endometritis
- infections of the uterus after abortion in the last 3 months
- conditions that promote the development of infection
- abnormal cervical cells
- cancer or suspected cancer of the cervix or uterus
- unexplained abnormal uterine bleeding
- abnormalities of the cervix or uterus, including fibroids, if they deform the uterine cavity
- active liver disease or liver tumor
Warnings and precautions
Before starting to use the Mirena system, you should consult a doctor.
Consult a doctor who will decide whether to remove or not remove the Mirena system if any of the following conditions occur for the first time while using the Mirena system:
- migraine, asymmetric visual field defects, or other symptoms that may be signs of transient cerebral ischemia (temporary blockage of blood flow to the brain)
- severe headache
- jaundice (yellowing of the skin, whites of the eyes, and/or nails)
- significant increase in blood pressure
- severe arterial disease, such as stroke or heart attack
- acute venous thromboembolic disease.
The Mirena system should be used with caution in women with congenital heart defects or heart valve defects that increase the risk of endocarditis.
In women with diabetes who use the Mirena system, blood glucose levels should be monitored.
Irregular bleeding may mask some symptoms and signs of endometrial polyps or cancer, and in these cases, diagnostic tests should be considered.
Medical examination/consultation
The examination before inserting the Mirena system may include a cervical smear as well as other tests, such as infection screening, including sexually transmitted diseases if necessary, and a pregnancy test. A gynecological examination should be performed to determine the position and size of the uterus.
The Mirena system is not a suitable contraceptive method for use in emergency situations (post-coital contraception, emergency contraception).
Infections
The applicator tube helps protect the Mirena system from microbial contamination during insertion. The Mirena applicator has been designed to reduce the risk of infection. Nevertheless, there is an increased risk of infection shortly after insertion and during the first month after insertion of the Mirena system. Pelvic inflammatory disease in women using the Mirena system is often associated with sexually transmitted diseases. The risk of infection increases with multiple sexual partners. Pelvic inflammatory disease must be properly treated, as it may affect fertility and increase the risk of ectopic pregnancy. The Mirena system should be removed in case of recurrent endometritis or pelvic inflammatory disease, if there are acute and severe inflammatory conditions, or if they do not resolve after a few days of treatment.
In extremely rare cases, shortly after insertion of the intrauterine therapeutic system, severe infection or sepsis (a very severe infection that can be fatal) may occur.
You should contact a doctor immediately if you experience persistent lower abdominal pain, elevated body temperature, pain during sexual intercourse, and unusual vaginal bleeding.
Expulsion
Uterine contractions during menstrual bleeding may sometimes cause the system to move or be expelled. This is more likely if the woman is overweight at the time of insertion or if she has had heavy menstrual bleeding in the past. If the system is not in place, it may not work as intended, and the risk of pregnancy increases. Expulsion of the system results in loss of contraceptive protection.
Possible symptoms of expulsion include vaginal bleeding or lower abdominal pain, but Mirena may also be expelled without symptoms. Since Mirena reduces menstrual bleeding, the intensity of these bleedings may be a sign of expulsion or displacement of the system.
It is recommended to check with your fingers (e.g., during bathing) whether the threads are in the correct position. See also section 3 "How to use Mirena - Self-examination of the correct position of the Mirena system". If symptoms occur that may indicate expulsion of the system or if the threads cannot be felt in the cervical area, you should use other contraceptive methods (such as condoms) and contact a doctor.
Perforation of the uterine wall
Perforation or damage to the uterine wall may occur, most often during insertion, although it may only be detected later. A Mirena system that is outside the uterine cavity is not effective in preventing pregnancy and should be removed as soon as possible. Surgical removal of the Mirena system may be necessary. The risk of perforation is higher in breastfeeding women and within 36 weeks postpartum; this risk may also be increased in women with a permanently retroverted uterus (retroversion of the uterus). If you suspect perforation of the uterine wall, you should consult a doctor and inform them that you have a Mirena system, especially if it is not the doctor who inserted the system.
Possible signs and symptoms of perforation may include:
- severe pain (like menstrual cramps) or pain stronger than expected
- severe bleeding (after insertion)
- pain or bleeding that lasts longer than a few weeks
- sudden changes in your periods
- pain during sexual intercourse
- inability to feel the Mirena threads (see section 3 "How to use Mirena - Self-examination of the correct position of the Mirena system")
Ectopic pregnancy
Getting pregnant while using the Mirena system is very unlikely. However, if a woman becomes pregnant while using the Mirena system, the risk of ectopic pregnancy is relatively increased. About 1 in 1000 women who properly used the Mirena system experienced an ectopic pregnancy within a year of using the system. This is less than in women who do not use any contraceptive methods (about 3 to 5 in 1000 women per year). In women who have had an ectopic pregnancy before, who have had tubal surgery, or who have had pelvic inflammatory disease, there is an increased risk of ectopic pregnancy. Ectopic pregnancy is a serious condition that requires immediate medical attention.
Symptoms that may indicate ectopic pregnancy and require immediate medical attention include:
- cessation of menstrual bleeding, followed by persistent bleeding or pain
- dull or severe lower abdominal pain
- typical symptoms of pregnancy with concurrent bleeding and dizziness.
Dizziness
Some women may experience dizziness after insertion of the Mirena system. This is a normal physiological reaction. The doctor will recommend resting for a while after insertion of the Mirena system.
Enlarged ovarian follicles surrounding the maturing egg cell in the ovary
The contraceptive properties of the Mirena system are related to its local action; therefore, in women of childbearing age, menstrual cycles are usually ovulatory, and ovulation occurs. Sometimes, the unruptured follicle does not regress for a while and may enlarge. In most cases, these enlarged follicles do not cause symptoms, although they may cause pelvic pain or pain during sexual intercourse. Such enlarged ovarian follicles usually resolve on their own, but they may also require medical intervention.
Mirena and other medicines
Since the mechanism of action of the Mirena system is primarily local, taking other medicines is unlikely to increase the risk of pregnancy during use of this system.
However, you should tell your doctor about all medicines you have taken recently, including those that are available without a prescription.
Pregnancy, breastfeeding, and fertility
Pregnancy
Mirena should not be used during pregnancy or if pregnancy is suspected.
Very rarely, a woman may become pregnant while the Mirena system is in place. However, if the Mirena system is displaced, contraceptive protection is reduced, and other contraceptive methods should be used until a doctor's visit.
During use of the Mirena system, some women may experience cessation of menstrual bleeding after a while. Absence of menstrual bleeding does not always mean pregnancy. If menstrual bleeding has stopped and other signs of pregnancy are present (e.g., nausea, fatigue, breast tenderness), you should consult a doctor for an examination and a pregnancy test.
If you become pregnant while using the Mirena system, you should contact a doctor immediately to have the system removed. Removal may cause miscarriage. However, leaving the Mirena system in place during pregnancy may increase the risk not only of miscarriage but also of preterm birth. If the Mirena system cannot be removed, you should discuss the benefits and risks of continuing the pregnancy with your doctor. If the pregnancy continues, it should be monitored closely by a doctor, and you should immediately report any symptoms such as abdominal cramps, abdominal pain, or fever to your doctor.
Mirena contains a hormone called levonorgestrel, and there have been individual reports of its effect on the genital organs of female infants exposed to levonorgestrel released from an intrauterine device left in the uterus.
Breastfeeding
Mirena can be used during breastfeeding. Levonorgestrel passes into breast milk in small amounts (about 0.1% of the levonorgestrel dose may pass into the infant's body through breast milk). After 6 weeks postpartum, use of the Mirena system has no harmful effect on the growth and development of the infant. It has not been shown that progestogen-only contraceptives affect the quantity or quality of milk.
Hormonal contraception is not recommended as a first-line method during breastfeeding; only non-hormonal methods are recommended. Progestogen-only contraceptives, such as the Mirena system, are second-line methods. The daily dose and blood levels of levonorgestrel are lower than with other hormonal contraceptives.
Fertility
Removal of the Mirena system restores normal fertility in women.
If you are pregnant, breastfeeding, or think you may be pregnant, or plan to become pregnant, you should consult a doctor or pharmacist before taking this medicine.
Driving and using machines
No effects of the Mirena system on the ability to drive or use machines have been observed.
Important information about some ingredients of Mirena
The T-shaped frame of the Mirena system contains barium sulfate, which allows the system to be visible on X-ray.
3. How to use Mirena
Efficacy of the Mirena system
The contraceptive efficacy of the Mirena system is the same as that of the most effective copper-containing intrauterine device. Clinical trials have shown about 2 pregnancies per 1000 women using the Mirena system in the first year.
In the treatment of heavy menstrual bleeding of unknown origin, the intensity of bleeding decreases after 3 months of using the Mirena system. In some women, menstrual bleeding may even stop.
When to insert the Mirena system
Starting to use the Mirena system
- Before inserting the Mirena system, you should ensure that you are not pregnant.
- The Mirena system should be inserted within 7 days of the start of menstrual bleeding. If the Mirena system is inserted during these days, it will work immediately and prevent pregnancy.
- If the Mirena system cannot be inserted within 7 days of the start of menstrual bleeding or if menstrual bleeding occurs at an unpredictable time, the system may be inserted on any day. In this case, you should not have sexual intercourse without using contraception from the last menstrual bleeding until the system is inserted, and you should have a negative pregnancy test result. Additionally, the Mirena system may not prevent pregnancy immediately and reliably. Therefore, you should use a barrier method of contraception (e.g., condoms) or abstain from sexual intercourse for the first 7 days after insertion (see section 3 "How to use Mirena - When to insert the Mirena system?").
Starting to use the Mirena system after childbirth
- The Mirena system can be inserted after childbirth when the uterus has returned to its normal size, but not earlier than 6 weeks postpartum (see section 2: "Important information before using Mirena - Perforation of the uterine wall").
- See also "Starting to use the Mirena system" above to find out what else you need to know about the timing of insertion.
Starting to use the Mirena system after miscarriage
The Mirena system can be inserted immediately after a miscarriage in the first trimester, provided there is no infection of the genital tract. The Mirena system will be effective immediately.
Replacing the Mirena system
The system can be replaced with a new one on any day of the menstrual cycle. The Mirena system will be effective immediately.
Switching from another contraceptive method (e.g., combined hormonal contraceptives, implant)
- The Mirena system can be inserted immediately if it is certain that the patient is not pregnant.
- If more than 7 days have passed since the start of menstrual bleeding, you should abstain from sexual intercourse or use additional contraceptive protection for the next 7 days.
How to insert the Mirena system
The Mirena system should only be inserted by a doctor or other qualified medical personnel with experience in inserting the system.
After performing a gynecological examination, a speculum is inserted into the vagina, and the cervix is flushed with an antiseptic solution. The intrauterine system is then inserted into the uterus using a thin, flexible plastic tube (applicator). If necessary, the cervix can be locally anesthetized before insertion.
Some people may experience pain and dizziness after insertion of the system. If these symptoms do not resolve within half an hour while the patient is in a lying position, it may indicate that the system has been inserted incorrectly. An examination should be performed, and if necessary, the system should be removed.
After insertion of the Mirena system, the patient should receive a reminder card from the doctor, which should be brought to each scheduled visit.
When to consult a doctor
The doctor should check the presence of the system within 4 to 12 weeks after its insertion and then regularly check the presence of the system at least once a year. The doctor will determine individually how often and what control tests should be performed. You should bring the reminder card for the patient, received from the doctor, to each scheduled visit.
Additionally, you should consult a doctor if:
- the threads in the vagina are not palpable
- the lower part of the system is palpable
- you suspect you are pregnant
- you experience persistent abdominal pain, fever, or unusual vaginal discharge
- you or your partner experience pain or discomfort during sexual intercourse
- you experience sudden changes in your menstrual cycle (e.g., scant or absent menstrual bleeding, and then persistent bleeding or pain)
Remind your doctor that you have a Mirena system, especially if it is not the doctor who inserted the system.
Duration of use of the Mirena system
The Mirena system prevents pregnancy (has a contraceptive effect) for 8 years after insertion. If you are using the Mirena system for this purpose, the system should be removed or replaced no later than 8 years after insertion.
The Mirena system is effective for 5 years after insertion in the treatment of heavy menstrual bleeding (of unknown origin). If you are using Mirena for this purpose, the system should be removed or replaced when heavy menstrual bleeding returns or no later than 8 years after insertion. If you wish, a new system can be inserted after removal of the previous one.
If you want to remove the Mirena system to become pregnant or for other reasons
A doctor can easily remove the system at any time, and you can become pregnant. Removal of the system is usually painless. After removal of the Mirena system, fertility returns.
Continuing contraception after removal of the system
If you do not plan to become pregnant, the Mirena system should not be removed after the 7th day of the menstrual cycle (unless other contraceptive methods are used, such as condoms) for at least 7 days before removal. If you have irregular menstrual periods or do not have menstrual periods, you should use mechanical contraceptive methods for at least 7 days before removal and until menstrual bleeding resumes. Alternatively, you can have a new system inserted immediately after removal of the previous one, and no additional protection is required. If you do not want to continue using the same method, you should ask your doctor for advice on other proven contraceptive methods.
Can you become pregnant after stopping the use of the Mirena system
Yes. Removal of the Mirena system does not disrupt fertility. You can become pregnant during the first menstrual cycle after removal of the Mirena system.
Does the Mirena system affect menstrual bleeding
Mirena affects the menstrual cycle. The system may cause various changes in menstrual bleeding, such as spotting (minor blood loss), shorter or longer menstrual bleeding, scant or heavy menstrual bleeding, or its absence.
In many women, during the first 3 to 6 months after insertion of the Mirena system, frequent spotting or minor bleeding occurs in addition to menstrual bleeding. In some women, menstrual bleeding may become heavier or longer than usual. You should inform your doctor, especially if these symptoms do not resolve.
Is the absence of menstrual bleeding normal
Yes, when using the Mirena system. The absence of menstrual bleeding is a sign of the effect of the hormone on the uterine lining. There is no monthly thickening of the uterine lining. Therefore, there is nothing to be expelled with menstrual blood. This does not have to be a sign of menopause or pregnancy. Hormone levels remain normal.
In fact, the absence of menstrual bleeding can be a great benefit for a woman's health.
Diagnosing pregnancy
Becoming pregnant while using the Mirena system is unlikely, even if menstrual bleeding does not occur.
If menstrual bleeding has not occurred for 6 weeks and this causes concern, a pregnancy test can be performed. If the result is negative, there is no need for further testing unless other symptoms of pregnancy are present, such as nausea, fatigue, or breast tenderness.
Can the Mirena system cause pain or discomfort
Some women experience pain (like menstrual cramps) for a few weeks after insertion of the system. You should consult a doctor or clinic again if you experience severe pain or if pain persists for more than 3 weeks after insertion of the Mirena system.
Effect of the Mirena system on sexual intercourse
Neither you nor your partner should feel the system during sexual intercourse. However, if you do feel it, you should avoid sexual intercourse until a doctor checks whether the system is still in the correct position.
How soon after insertion of the system can you have sexual intercourse
To allow your body to rest, it is best to wait about 24 hours after insertion of the system before having sexual intercourse. Depending on when in your menstrual cycle the Mirena system is inserted, you may need to use emergency contraception (e.g., condoms) or abstain from sexual intercourse for the first 7 days after insertion (see section 3 "How to use Mirena - When to insert the Mirena system?").
Using tampons or menstrual cups
It is recommended to use sanitary pads. If you use tampons or menstrual cups, you should change them carefully to avoid pulling on the Mirena system threads. If you think the Mirena system has been displaced from its correct position (see "When to consult a doctor" with possible symptoms), you should avoid sexual intercourse or use mechanical contraception (such as condoms) and consult a doctor.
What happens if the Mirena system is expelled
Rarely, but it is possible, that the Mirena system may be expelled without your knowledge during menstrual bleeding. If menstrual bleeding is heavier than usual, it may indicate that the Mirena system has been expelled through the vagina. It is also possible that the Mirena system is partially expelled from the uterus (you or your partner may notice this during sexual intercourse). If the Mirena system is completely or partially expelled, it does not provide contraceptive protection.
Self-examination of the correct position of the Mirena system
You can check yourself whether the threads of the system are in the correct position. To do this, you should carefully insert your finger into the vagina and check for the presence of the threads near the cervix.
Do not pull on the threads, as this can cause the system to be accidentally removed. If you do not feel the threads, it may indicate that the system has been expelled from the uterus or that the uterine wall has been perforated. You should then use mechanical contraception (e.g., condoms) and consult a doctor.
4. Possible side effects
Like all medicines, Mirena can cause side effects, although not everybody gets them.
In addition to the possible side effects listed in other sections (e.g., section 2 "Important information before using Mirena"), the following side effects are possible, divided by body system and frequency of occurrence:
Very common side effects:may occur in more than 1 in 10 people
Reproductive system and breast disorders
- uterine or vaginal bleeding, including spotting, infrequent periods, or their absence
- mild ovarian cysts (see section 2: "enlarged ovarian follicles")
Common side effects:may occur in less than 1 in 10 people
Psychiatric disorders
- depressed mood or depression
- nervousness
- decreased libido
Nervous system disorders
- headache
Vascular disorders
- dizziness
Gastrointestinal disorders
- abdominal pain
- nausea
Skin and subcutaneous tissue disorders
- acne
Musculoskeletal and connective tissue disorders
- back pain
Reproductive system and breast disorders
- pelvic pain
- painful menstruation
- vaginal discharge
- vulvovaginitis
- breast tenderness
- breast pain
- expulsion of the intrauterine system
Investigations
- weight gain
Uncommon side effects:may occur in less than 1 in 100 people
Nervous system disorders
- migraine
Gastrointestinal disorders
- abdominal distension
Skin and subcutaneous tissue disorders
- hirsutism (male-type hair growth in women)
- hair loss (alopecia)
- pruritus (severe itching)
- rash (skin inflammation)
- chloasma (yellow-brown spots on the skin) or intense skin discoloration
Reproductive system and breast disorders
- perforation (puncture) of the uterus
- pelvic inflammatory disease (infection of the upper female reproductive organs)
- endometritis (inflammation of the uterine lining)
- cervicitis - normal class II Papanicolaou smear in cytological examination (cervicitis)
General disorders and administration site conditions
- edema
Rare side effects:may occur in less than 1 in 1000 people
Skin and subcutaneous tissue disorders
- urticaria
- angioedema
If you become pregnant while using the Mirena system, there is a risk of ectopic pregnancy (see section 2: "Ectopic pregnancy").
After insertion of the intrauterine therapeutic system, cases of sepsis (a very severe infection that can be fatal) have been reported.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder or parallel importer.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Mirena
Store out of sight and reach of children.
Storage: No special storage temperature instructions.
Store in the original packaging.
Do not insert Mirena after the expiration date stated on the packaging. The expiration date refers to the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Mirena contains
- The active substance of Mirena is levonorgestrel. One intrauterine therapeutic system contains 52 mg of levonorgestrel.
- The other ingredients of the medicine are: polydimethylsiloxane elastomer, polydimethylsiloxane tubing (containing 30-40% anhydrous colloidal silica); T-body composed of: polyethylene (containing 20-24% barium sulfate); threads composed of: polyethylene, black iron oxide (E 172) less than 1%.
What Mirena looks like and contents of the packaging
Package size: one sterile-packaged intrauterine therapeutic system for intrauterine use.
For more detailed information, you should contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Romania, the country of export:
Bayer AG
Kaiser-Wilhelm-Allee 1
51373 Leverkusen
Germany
Manufacturer:
Bayer Oy
Pansiontie 47
20210 Turku
Finland
Parallel importer:
Allpharm Sp. z o.o. sp.k.
ul. M. Zdziechowskiego 11/4
02-659 Warsaw
Repackaged by:
CEFEA Sp. z o.o. Sp.
komandytowa
ul. Działkowa 56
02-234 Warsaw
Synoptis Industrial Sp. z o.o.
ul. Forteczna 35-37
87-100 Toruń
Shiraz Productions Sp. z o.o.
ul. Tymiankowa 24/28
95-054 Ksawerów
Marketing authorization number in Romania, the country of export:7842/2015/01
Parallel import authorization number: 393/24
If you have any further questions, you should consult a doctor or pharmacist.
Date of approval of the leaflet: 14.11.2024
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Bayer AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MirenaDosage form: System, 19.5 mg/systemActive substance: plastic IUD with progestogenManufacturer: BAYER OyPrescription requiredDosage form: System, 52 mg (20 mcg/24h)Active substance: plastic IUD with progestogenPrescription requiredDosage form: System, 52 mg (20 mcg/24 h)Active substance: plastic IUD with progestogenPrescription required
Alternatives to Mirena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mirena in Ukraine
Alternative to Mirena in Spain
Online doctors for Mirena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mirena – subject to medical assessment and local rules.