VAXIGRIP TETRA SUSPENSION INYECTABLE EN JERINGA PRECARGADA

Cómo usar VAXIGRIP TETRA SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el usuario
Vaxigrip Tetra, suspensión inyectable en jeringa precargada
Vacuna antigripal tetravalente (virus fraccionados, inactivados)
Lea todo el prospecto detenidamente antes de que usted o su hijo sean vacunados porque contiene información importante para usted o su hijo.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted o a su hijo y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si usted o su hijo experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Vaxigrip Tetra y para qué se utiliza
- Qué necesita saber antes de que usted o su hijo empiecen a usar Vaxigrip Tetra
- Cómo usar Vaxigrip Tetra
- Posibles efectos adversos
5 Conservación de Vaxigrip Tetra
- Contenido del envase e información adicional
1. Qué es Vaxigrip Tetra y para qué se utiliza
Vaxigrip Tetra es una vacuna.
Esta vacuna se le administra a usted o a su hijo desde los 6 meses de edad, está indicada para protegerle a usted o a su hijo frente a la gripe.
Cuando se inyecta a una persona Vaxigrip Tetra, el sistema inmunológico (el sistema de las defensas naturales del cuerpo) producirá protección (anticuerpos) frente a la infección.
Cuando se administra la vacuna durante el embarazo, además de proteger a la mujer embarazada también protege a su bebé desde el nacimiento hasta los 6 meses de edad a través de la trasmisión de la protección de la madre al bebé durante el embarazo (ver también las secciones 2 y 3).
Ninguno de los componentes de la vacuna puede causar gripe.
El uso de Vaxigrip Tetra se debe basar en las recomendaciones oficiales.
La gripe es una enfermedad que se puede extender rápidamente y es causada por diferentes tipos de cepas que pueden cambiar cada año. Debido a este cambio potencial en las cepas circulantes cada año, así como la duración de la protección prevista por la vacuna, la vacunación se recomienda todos los años. Hay mayor riesgo de contagiarse de gripe durante los meses de frío entre octubre y marzo. Si usted o su hijo no se vacunaron en otoño es posible vacunarse hasta la primavera ya que usted o su hijo corren el riesgo de infectarse por la gripe durante ese periodo. Su médico podrá recomendarle la mejor fecha para vacunarse.
El objetivo de Vaxigrip Tetra es protegerle a usted o a su hijo frente a las cuatro cepas del virus contenido en la vacuna después de unas 2 a 3 semanas de la inyección. El periodo de incubación de la gripe es de unos días de modo que si usted o su hijo se exponen a la gripe inmediatamente antes o después de la vacunación podría desarrollar la enfermedad.
La vacuna no le protegerá a usted o su hijo frente al resfriado común incluso si algunos de los síntomas son similares a la gripe.
2. Qué necesita saber antes de empezar a usar Vaxigrip Tetra
Para asegurarse de que Vaxigrip Tetra es adecuado para usted o su hijo, es importante que consulte a su médico o farmacéutico si alguno de los puntos descritos a continuación le afectan a usted o a su hijo. Si hay algo que usted no entiende, consulte a su médico o farmacéutico para que se lo aclare.
No use Vaxigrip Tetra
- Si usted o su hijo son alérgicos a:
- Los principios activos o
- A cualquiera de los demás componentes de esta vacuna (enumerados en la sección 6), o
- A cualquiera de los componentes que pueden estar presentes en cantidades mínimas, como huevos (ovoalbúmina o proteínas de pollo), neomicina, formaldehído u octoxinol-9.
- Si usted o su hijo padecen una enfermedad que se acompañe de fiebre alta o moderada o una enfermedad aguda, en este caso se retrasará la vacunación hasta que se hayan recuperado.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Vaxigrip Tetra.
Consulte a su médico antes de vacunarse si usted o su hijo tienen:
- Una respuesta inmunológica debilitada (inmunodeficiencia o tratamiento con medicamentos que afecten al sistema inmunológico),
- Problemas de sangrado o se producen hematomas con facilidad.
Su médico decidirá si usted o su hijo deben recibir la vacuna. Antes o después de cualquier inyección, podría producirse un desmayo (especialmente en los adolescentes), por lo que debe informar a su médico o enfermero si usted o su hijo se han desmayado en anteriores ocasiones tras la administración de una inyección.
Como todas las vacunas, Vaxigrip Tetra puede no proteger totalmente a todas las personas que se vacunan.
No todos los bebés menores de 6 meses de edad nacidos de mujeres embarazadas vacunadas durante el embarazo estarán protegidos.
Si, por alguna razón, se les practica un análisis de sangre a usted o a su hijo a los pocos días de la vacunación frente a la gripe, por favor informen a su médico. Esto es porque se han observado resultados falsos positivos en pruebas serológicas en algunos pacientes vacunados recientemente.
Niños
No se recomienda el uso de Vaxigrip Tetra en niños menores de 6 meses de edad.
Uso de Vaxigrip Tetraconotros medicamentos
Informe a su médico o farmacéutico si usted o su hijo están utilizando, han utilizado recientemente o pudieran tener que utilizar cualquier otro medicamento o vacuna.
- Vaxigrip Tetra se puede administrar al mismo tiempo que otras vacunas, en diferentes extremidades.
- La respuesta inmunológica puede disminuir en el caso de tratamientos inmunosupresores, tales como los corticoesteroides, los medicamentos citotóxicos o la radioterapia.
Embarazo ylactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada, consulte a su médico o farmacéutico antes de utilizar esta vacuna.
Vaxigrip Tetra se puede utilizar en todas las fases del embarazo.
Vaxigrip Tetra se puede usar durante el periodo de lactancia.
Su médico/farmacéutico podrá decidir si usted debe ser vacunado con Vaxigrip Tetra.
Conducción y uso de máquinas
Vaxigrip Tetra tiene una influencia nula o insignificante sobre la capacidad de conducir y usar máquinas.
Vaxigrip Tetra contiene potasio y sodio
Este medicamento contiene menos de 1 mmol de potasio (39 mg) y menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente "exento de potasio" y "exento de sodio".
3. Cómo usar Vaxigrip Tetra
Posología
Adultos: 1 dosis de 0,5 ml.
Uso en niños
Los niños desde los 6 meses a los 17 años de edad reciben una dosis de 0,5 ml.
Si su hijo es menor de 9 años y no ha sido previamente vacunado frente a la gripe, se debe administrar una segunda dosis de 0,5 ml después de un intervalo de al menos 4 semanas.
Si está embarazada, una dosis de 0,5 ml administrada durante el embarazo puede proteger a su bebé desde el nacimiento hasta los 6 meses de edad. Para mayor información pregunte a su médico o farmacéutico.
Cómo se administra Vaxigrip Tetra
Su médico o enfermero le administrará la dosis recomendada de la vacuna con una inyección en el músculo o bajo la piel.
Si usted o su hijo reciben más Vaxigrip Tetra del que deberían
En algunos casos, se administró involuntariamente más dosis de la recomendada.
En estos casos, cuando se notificaron acontecimientos adversos, estos estaban en línea con los descritos tras la administración de la dosis recomendada (ver Sección 4).
Si usted tiene cualquier duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Contacte inmediatamente con su médico o profesional sanitario o diríjase inmediatamente a Urgencias del hospital más cercano si usted o su hijo experimentan reacciones alérgicas (notificadas como raras: pueden afectar hasta 1 de cada 1.000 personas) que pueden ser amenazantes para la vida. Los síntomas pueden incluir erupción cutánea, picor, urticaria, enrojecimiento, dificultad para respirar, falta de aliento, hinchazón de la cara, labios, garganta, o lengua, piel fría y pegajosa, palpitaciones, mareos, debilidad o desfallecimiento.
Otros efectos adversos notificados en adultos y personas mayores
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza, dolor muscular (mialgia), malestar general (malestar) (1), dolor en el lugar de la inyección.
(1) Frecuente en personas mayores
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Fiebre (2), escalofríos, reacciones en el lugar de inyección: enrojecimiento (eritema), hinchazón, dureza (induración).
(2) Poco frecuente en personas mayores
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- Mareos (3), diarrea, sensación de enfermedad (náuseas) (4), fatiga, reacciones en el lugar de inyección: hematomas (equimosis), picor (prurito) y calor.
(3) Rara en adultos (4) Rara en personas mayores
- Sofocos: sólo observados en personas mayores.
- Hinchazón de los ganglios del cuello, la axila o la ingle (linfadenopatía): sólo observado en adultos.
Raras (pueden afectar hasta 1 de cada 1.000 personas)
- Anomalías en la percepción del tacto, dolor, calor y frío (parestesia), somnolencia, aumento de la sudoración (hiperhidrosis), cansancio inusual y debilidad (astenia), enfermedad de tipo gripal.
- Dolor en las articulaciones (artralgia), molestias en el lugar de inyección: sólo observados en adultos.
Otros efectos adversos notificados en niños de entre 3 y 17 años de edad
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza, dolor muscular (mialgia), malestar general (malestar), escalofríos (5), reacciones en el lugar de inyección: dolor, hinchazón, enrojecimiento (eritema) (5), dureza (induración) (5).
(5) Frecuente en niños de 9 a 17 años
Frecuentes (pueden afectar hasta 1 de cada 10 personas)
- Fiebre, hematoma en el lugar de la inyección (equimosis).
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas) en niños entre 3 y 8 años de edad:
- Disminución temporal en el número de ciertas células de la sangre llamadas plaquetas; cuando el número es bajo puede dar lugar a la formación excesiva de hematomas en la piel o sangrado (trombocitopenia transitoria): sólo observado en un niño de 3 años de edad
- Quejidos, inquietud
- Mareos, diarrea, vómitos, dolor abdominal superior, dolor en las articulaciones (artralgia), fatiga, calor en el lugar de la inyección.
Poco frecuentes (pueden afectar a 1 de cada 100 personas) en niños desde los 9 a los 17 años de edad:
- Diarrea, picor en el lugar de la inyección (prurito).
Otros efectos adversos notificados en niños entre 6 y 35 meses de edad
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Vómitos (1), dolor muscular (mialgia) (2), irritabilidad (3), pérdida de apetito (3), malestar general (malestar) (2), fiebre.
(1) Poco frecuente en niños de 24 a 35 meses de edad (2) Rara en niños menores de 24 meses de edad
(3) Rara en niños de 24 a 35 meses de edad.
- Reacciones en el lugar de inyección: dolor/dolor a la palpación, enrojecimiento (eritema).
- Dolor de cabeza: solo observado en niños a partir de los 24 meses de edad.
- Somnolencia, llanto anormal: solo observados en niños menores de 24 meses de edad.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Escalofríos: solo observado en niños desde los 24 meses de edad en adelante.
- Reacciones en el lugar de inyección: endurecimiento (induración), hinchazón, hematoma (equimosis)
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Diarrea, hipersensibilidad.
Raras (pueden afectar hasta 1 de cada 1.000 personas):
- Enfermedad de tipo gripal, reacciones en el lugar de la inyección: erupción, prurito (picazón).
En niños de 6 meses a 8 años de edad que reciben 2 dosis, los efectos adversos son similares tanto después de la primera dosis como después de la segunda dosis. Algunos efectos adversos pueden ocurrir después de la administración de la segunda dosis en niños de 6 meses a 35 meses de edad.
Estos efectos adversos, cuando se observaron, normalmente ocurrieron durante los 3 primeros días siguientes a la vacunación y desaparecieron, por sí mismos, entre 1 y 3 días tras iniciarse. La mayoría de estos efectos adversos fueron de intensidad leve.
En general, los efectos adversos fueron menos frecuentes en personas mayores que en adultos y niños.
Los siguientes efectos adversos han sido observados después de administrar Vaxigrip. Estos efectos adversos también pueden ocurrir con Vaxigrip Tetra:
- Dolor situado en la ruta del nervio (neuralgia), ataques (convulsiones), trastornos neurológicos que pueden resultar en rigidez en el cuello, confusión, entumecimiento, dolor y debilidad de las extremidades, pérdida del equilibrio, pérdida de reflejos, parálisis de una parte o de todo el cuerpo (encefalomielitis, neuritis, síndrome de Guillain-Barré).
- Inflamación de los vasos sanguíneos (vasculitis) que pueden dar lugar a erupciones cutáneas y en muy raras ocasiones a problemas pasajeros de riñón.
- Trombocitopenia transitoria, linfadenopatía, parestesia en otros grupos de edad distintos de los descritos anteriormente para estos efectos secundarios.
Comunicación de efectos adversos
Si usted o su hijo experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Vaxigrip Tetra
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice esta vacuna después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (2ºC - 8ºC). No congelar. Conservar la jeringa en el embalaje exterior para protegerla de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Vaxigrip Tetra
- Los principios activos son: Virus de la gripe (fraccionados, inactivados) de las siguientes cepas*:
- Cepa similar a A/Victoria/4897/2022 (H1N1)pdm09: (IVR-238)........15 microgramos HA**
- Cepa similar a A/Croacia /10136RV/2023 (H3N2): (X-425)..............15 microgramos HA**
- Cepa similar a B/Austria/1359417/2021: B/Michigan/01/2021 ..…....15 microgramos HA**
- B/Phuket/3073/2013..............................................................................15 microgramos HA**
Por dosis de 0,5 ml
- cultivados en huevos de gallina embrionados procedentes de pollos sanos
** hemaglutinina
Esta vacuna cumple con las recomendaciones de la Organización Mundial de la Salud (Hemisferio Norte) y la decisión de la Unión Europea para la campaña 2025-2026.
- Los demás componentes son una solución tampón que contiene cloruro de sodio, fosfato de disodio dihidrato, fosfato dihidrógeno de potasio, cloruro de potasio y agua para preparaciones inyectables.
Algunos componentes tales como huevos (ovoalbúmina, proteínas de pollo), neomicina, formaldehído o octoxinol-9 pueden estar presentes en cantidades muy pequeñas (ver sección 2).
Aspecto del producto y contenido del envase
Después de agitarla cuidadosamente, la vacuna es un líquido ligeramente blanquecino y opalescente.
Vaxigrip Tetra se presenta en una jeringa precargada que contiene 0,5 ml de suspensión inyectable, con aguja fija o sin aguja (envases de 1, 10 o 20) o con aguja de seguridad (en envases de 1 o 10).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
Francia
Responsable de la fabricación:
El fabricante responsable de la liberación de los lotes es:
Sanofi Winthrop Industrie
1541 avenue Marcel Mérieux
69280 Marcy l’Etoile
Francia
o
Sanofi Winthrop Industrie
Voie de l’Institut – Parc Industriel d’Incarville
B.P 101
27100 Val de Reuil
Francia
o
SANOFI-AVENTIS ZRT
Campona U.1 (Harbor Park )
Budapest 1225
Hungría
Representante local
sanofi-aventis, S.A.
C/ Rosselló i Porcel, 21
08016 Barcelona
España
Tel: +34 93 485 94 00
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Estado Miembro | Nombre |
| VaxigripTetra Injektionssuspension in einer Fertigspritze VaxigripTetra injekcine suspensija užpildytame švirkšte VaxigripTetra Vaxigriptetra Vaxigrip Tetra suspension injectable en seringue préremplie Vaxigrip Tetra Quadrivalent influenza vaccine (split virion, inactivated) |
Fecha de la última revisión de esteprospecto: Mayo 2025
Otras fuentes de información
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el cartonaje. También puede acceder a esta información en la siguiente dirección de internet: https://vaxigriptetra-nh.info.sanofi
--------------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a médicos o profesionales del sector sanitario:
Como con todas las vacunas inyectables, se debe disponer del tratamiento médico y la supervisión apropiada en el caso de que ocurra un episodio anafiláctico tras la administración de la vacuna.
La vacuna debe alcanzar la temperatura ambiente antes de su utilización.
Agitar antes de usar.
La vacuna no se debe utilizar si presenta partículas extrañas en la suspensión.
No se debe mezclar con otros medicamentos en la misma jeringa.
Esta vacuna no se debe inyectar directamente en ningún vaso sanguíneo.
Ver también sección 3. Cómo usar Vaxigrip Tetra.
Instrucciones de uso de la aguja de seguridad con la jeringa precargada Luer Lock:
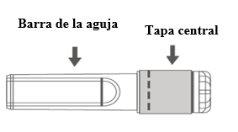
Imagen A: Aguja de seguridad (dentro de la barra) | Imagen B: Componentes de la aguja de seguridad (preparada para su uso) |
|
|
Paso 1:Para fijar la aguja a la jeringa, retire la tapa central para exponer la barra de la aguja, y girar suavemente la aguja en el adaptador Luer Lock de la jeringa hasta que note una ligera resistencia. |
Paso 2:Extraiga el protector de la aguja de seguridad. La aguja está cubierta por el dispositivo de seguridad y el protector. |
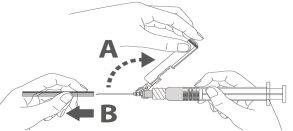
Paso 3: A:Separe el dispositivo de seguridad de la aguja hacia el cuerpo de la jeringa en el ángulo que se muestra. B:Retire el protector en linea recta. |
|
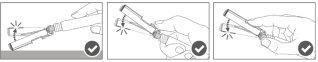
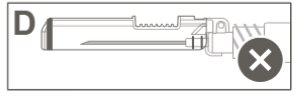
Paso 4:Una vez finalizada la inyección, bloquee (active) el dispositivo de seguridad utilizando una de las tres técnicas ilustradas (3) con una sola mano: activación con una superficie, con el pulgar o con el dedo índice. Nota: La activación se verifica mediante un sonido "clic" y/o táctil. |
|
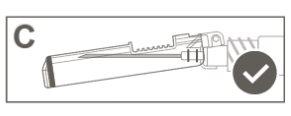
Paso 5:Inspeccionar visualmente la activación del dispositivo de seguridad. El dispositivo de seguridad debe estar completamente bloqueado (activado)como se muestra en la figura C. La figura D muestra que el dispositivo de seguridad NO está completamente bloqueado (no activado). |
|
Precaución: No intente desbloquear (desactivar) el dispositivo de seguridad forzando la aguja fuera del dispositivo de seguridad.> |
La eliminación de las vacunas no utilizadas y de todos los materiales que hayan estado en contacto con ella se realizará de acuerdo con la normativa local.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VAXIGRIP TETRA SUSPENSION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 3,75 microgramosPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, 0,5 mlPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Sanofi Winthrop IndustrieRequiere recetaForma farmacéutica: INYECTABLE, 60 microgramos de HAPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para VAXIGRIP TETRA SUSPENSION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VAXIGRIP TETRA SUSPENSION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes