ТОРЕНС 10 000 МО/мл оральні краплі в розчині
Запитайте лікаря про рецепт на ТОРЕНС 10 000 МО/мл оральні краплі в розчині

Інструкція із застосування ТОРЕНС 10 000 МО/мл оральні краплі в розчині
Введення
Опис: інформація для користувача
Торенс 10 000МО/мл краплі оральної розчини
колекальциферол (вітамін Д3)
Прочитайте уважно весь опис перед початком прийому цього лікарського засобу, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь сумніви, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки вам, і не слід давати його іншим людям, навіть якщо вони мають такі самі симптоми, як у вас, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не перелічені в цьому описі. Див. розділ 4.
Зміст опису:
- Що таке Торенс і для чого він використовується
- Що потрібно знати перед початком прийому Торенсу
- Як приймати Торенс
- Можливі побічні ефекти
- Збереження Торенсу
- Зміст упаковки та додаткова інформація
1. Що таке Торенс і для чого він використовується
Торенс краплі оральної розчини містять колекальциферол (вітамін Д3). Вітамін Д3 можна знайти в деяких продуктах харчування і виробляється організмом, коли шкіра піддається дії сонячного світла. Вітамін Д сприяє абсорбції кальцію в нирках і кишечнику, допомагаючи утворенню кісток. Дефіцит вітаміну Д є основною причиною ракіту (недостатньої мінералізації кісток у дітей) і остеомаляції (недостатньої мінералізації кісток у дорослих).
Торенс краплі оральної розчини використовуються для профілактики і лікування дефіциту вітаміну Д3 у дорослих, підлітків і дітей з ідентифікованим ризиком дефіциту вітаміну Д3.
Торенс краплі оральної розчини також можуть використовуватися як допоміжний засіб до спеціальної медикаментозної терапії при втраті маси кісток.
2. Що потрібно знати перед початком прийому Торенсу
Не приймайтеТоренс
- якщо ви алергічні на вітамін Д3 або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6);
- якщо у вас є високі рівні кальцію в крові (гіперкальціємія) або в сечі (гіперкалціурія);
- якщо у вас є камені в нирках (нефролітіаз) або важка ниркова недостатність;
- якщо у вас є високі рівні вітаміну Д в крові (гіпервітаміноз Д).
Попередження і застереження
Проконсультуйтеся з вашим лікарем або фармацевтом перед початком прийому Торенсу, якщо
- ви приймаєте деякі лікарські засоби, призначені для лікування серцевих захворювань (наприклад, глікозиди, такі як дигоксин).
- ви страждаєте на саркоїдоз (автоімунне захворювання, яке може спричинити підвищення рівнів вітаміну Д в організмі).
- ви приймаєте лікарські засоби, які містять вітамін Д, або споживаєте продукти харчування, збагачені вітаміном Д.
- ви, ймовірно, будете приймати сонячні ванні під час прийому Торенсу.
- ви приймаєте інші добавки, які містять кальцій. Ваш лікар повинен контролювати рівні кальцію, щоб забезпечити, що вони не будуть надто високими під час лікування Торенсом.
- ви страждаєте на ниркове захворювання або пошкодження. Ваш лікар повинен контролювати рівні кальцію в крові та сечі.
лікар повинен контролювати рівні кальцію в крові за допомогою лабораторних аналізів, якщо щоденне споживання вітаміну Д3 перевищує 1000 МО під час тривалого часу
ВикористанняТоренсуіз іншими лікарськими засобами
Повідомте вашого лікаря або фармацевта, якщо ви приймаєте, нещодавно приймали або можете приймати інші лікарські засоби. Це особливо важливо, якщо ви приймаєте:
- лікарські засоби, призначені для лікування серцевих захворювань або нирок, такі як глікозиди (наприклад, дигоксин) або діуретики (наприклад, бендрофлуметазид). Коли ці лікарські засоби використовуються одночасно з вітаміном Д3, вони можуть спричинити значне підвищення рівнів кальцію в крові та сечі.
- лікарські засоби, які містять вітамін Д3 або продукти харчування, збагачені вітаміном Д3, такі як деякі види молока, збагаченого вітаміном Д3.
- актиноміцин (лікарський засіб, який використовується для лікування деяких видів раку) і антимікотичні засоби імідазолу (наприклад, клотримазол і кетоконазол, які використовуються для лікування захворювань, спричинених грибами). Ці лікарські засоби можуть перешкоджати обробці вітаміну Д3 в організмі.
- наступні лікарські засоби, оскільки вони можуть перешкоджати дії або абсорбції вітаміну Д3:
- протиепілептичні лікарські засоби (антikonvulsivanti), барбітурати;
- глюкокортикоїди (стероїдні гормони, такі як гідрокортизон або преднізолон), оскільки вони можуть зменшити дію вітаміну Д3;
- лікарські засоби, які знижують рівень холестерину в крові (наприклад, коlestiramina або коlestipol);
- деякі лікарські засоби, які використовуються для зниження ваги шляхом зниження абсорбції жиру (наприклад, орлістат);
- деякі проносні засоби (наприклад, рідка парафін).
ВикористанняТоренсуіз харчовими продуктами, напоями та алкоголем
Ви повинні приймати Торенс переважно під час однієї з основних їжі, щоб допомогти абсорбції вітаміну Д3. Ви можете приймати його окремо або змішувати краплі з холодною або теплою їжею, щоб полегшити прийом. Для більшої інформації див. розділ 3 «Як приймати Торенс».
Вагітність, лактація та фертильність
Якщо ви вагітні або перебуваєте в період лактації, вважаєте, що можете бути вагітні або плануєте завагітніти, проконсультуйтеся з вашим лікарем або фармацевтом перед прийомом цього лікарського засобу.
Торенс повинен прийматися під час вагітності та лактації тільки за рекомендацією вашого лікаря.
Передозування вітаміну Д повинні бути уникнуті під час вагітності, оскільки тривала гіперкальціємія може спричинити затримку розвитку дитини та інші порушення.
Водіння транспортних засобів та використання машин
Інформація про можливі ефекти цього лікарського засобу на здатність водити транспортні засоби обмежена. Однак не очікується, що він буде впливати на здатність водити транспортні засоби та використання машин.
3. Як приймати Торенс
Слідуйте точно інструкціям з прийому цього лікарського засобу, вказаним вашим лікарем. У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом знову.
Витрясайте перед використанням.
Торенс повинен прийматися переважно під час основних їжі.
Цей лікарський засіб має смак оливкової олії. Торенс можна приймати окремо або змішуючи кількість крапель, призначену лікарем, з невеликою кількістю холодної або теплої їжею безпосередньо перед прийомом. Ви повинні забезпечити прийом повної дози.
Використання у дорослих
Рекомендована доза для дефіциту вітаміну Ді як допоміжний засіб до спеціальної медикаментозної терапії при втраті маси кісток (остеопороз) становить 3-4 краплі (600 МО - 800 МО) на добу.
Для лікування дефіциту вітаміну Д доза зазвичай становить 4 краплі (800 МО) на добу. Вищі дози повинні бути кориговані залежно від рівнів серичного 25-гідроксиколекальциферолу (25(ОН)Д), тяжкості захворювання та реакції пацієнта на лікування.
Добова доза не повинна перевищувати 4000 МО (20 крапель на добу).
Використання у дітей та підлітків
Для профілактики дефіциту вітаміну Д у дітей (від 0 до 11 років) з ідентифікованим ризиком дефіциту вітаміну Д рекомендується доза 2 краплі (400 МО) на добу. Для профілактики дефіциту вітаміну Д у підлітків (від 12 до 18 років) з ідентифікованим ризиком дефіциту вітаміну Д рекомендується доза 3-4 краплі (600-800 МО) на добу.
Для лікування дефіциту вітаміну Д у дітей (від 0 до 11 років) та підлітків (від 12 до 18 років) доза повинна бути коригована залежно від рівнів серичного 25-гідроксиколекальциферолу (25(ОН)Д), тяжкості захворювання та реакції пацієнта на лікування. Добова доза не повинна перевищувати 1000 МО на добу для дітей молодше 1 року, 2000 МО на добу для дітей від 1 року до 10 років, і 4000 МО на добу для підлітків
У дітей Торенс можна змішувати з невеликою кількістю дитячого харчування, йогуртом, молоком, сиром або іншими молочними продуктами. Не додавайте Торенс до бутелів з молоком або інших контейнерів з харчовими продуктами, які дитина не буде споживати повністю безперервно, щоб уникнути того, що дитина не прийме повної дози. Ви повинні забезпечити прийом повної дози дитиною. У разі дітей, які закінчили період лактації, призначена доза повинна бути прийнята під час основних їжі.
Не зберігайте жоден продукт або харчовий засіб, який містить Торенс, для використання пізніше або в наступній їжі.
Використання під час вагітності та лактації
Рекомендована доза становить 400 МО/добу (2 краплі). Можуть бути необхідні вищі дози після підтвердження дефіциту вітаміну Д, але не слід приймати більше, ніж рекомендує ваш лікар.
Інструкції з використання для флакона з дозувальним піпеткою:
Упаковка містить флакон і дозувальний піпет. Флакон закрито дитячою кришкою. Піпет захищено пластиковим кільцем. Для використання лікарського засобу витрясайте флакон перед використанням і слідуйте інструкціям:
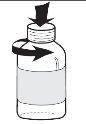
- для відкриття флакона натисніть на кришку і поверніть її одночасно (див. Фігура 1);
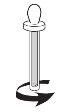
- відкрутіть пластикову упаковку, яка містить дозувальний піпет (див. Фігура 2);
- вставте дозувальний піпет у флакон, щоб витягти вміст. Положіть у ложку кількість крапель, призначену лікарем;
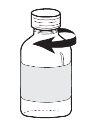
- для закриття флакона вийміть дозувальний піпет з флакона і поверніть оригінальну кришку на місце (див. Фігура 3);
- Осторожно поверніть дозувальний піпет у пластикову упаковку і закрутіть її.
- Помістіть флакон і дозувальний піпет у оригінальну коробку.
Фігура 1 |
Фігура 2 |
Фігура 3 |
Інструкції з використання дозувального піпета:
Упаковка містить дозувальний піпет.
Флакон закрито дитячою кришкою.
Наступні інструкції вказують на відкриття і використання:
- для відкриття флакона з дозувальним піпетом натисніть і поверніть пластикову кришку одночасно;
- помістіть флакон у вертикальне положення вниз і відміряйте кількість крапель, призначену лікарем, у ложку;
- після закінчення прийому крапель поверніть флакон з дозувальним піпетом у вертикальне положення
- для закриття флакона з дозувальним піпетом поверніть дитячу кришку;
- помістіть флакон у оригінальну паперову коробку.
Якщо ви прийняли більше Торенсу, ніж потрібно
Перестаньте приймати лікарський засіб і негайно зверніться до вашого лікаря або фармацевта, якщо ви або ваша дитина прийняли більшу кількість цього лікарського засобу, ніж призначено лікарем. Якщо це неможливо, зверніться до найближчої лікарні і принесіть упаковку з лікарським засобом.
Також ви можете звернутися до Токсикологічної служби, телефон 91 562 04 20, вказавши назву лікарського засобу і кількість, прийняту.
Найбільш часті симптоми передозування: нудота, блювота, надмірна спрага, надмірна виділення сечі протягом 24 годин, запор, зневоднення і підвищені рівні кальцію в крові та сечі (гіперкальціємія та гіперкалціурія) у клінічних аналізах.
Якщо ви забули прийнятиТоренс
Якщо ви забули прийняти одну дозу Торенсу, прийміть наступну якнайшвидше. Після цього прийміть наступну дозу в звичайний час. Однак, якщо це дуже близько до часу наступної дози, не прийміть забуту дозу і прийміть наступну дозу в звичайний час.
Не прийміть подвійну дозу для компенсації пропущених доз.
Якщо у вас є якісь інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і будь-який інший лікарський засіб, цей лікарський засіб може спричинити побічні ефекти, хоча не всі люди їх відчувають.
Можливі побічні ефекти, пов'язані з використанням Торенсу, можуть бути:
Нечасто (впливають до 1 особи з 100):
- надмірна кількість кальцію в крові (гіперкальціємія);
- надмірна кількість кальцію в сечі (гіперкалціурія).
Рідко (впливають до 1 особи з 1000):
- шкірний висип
- свербіж
- кропив'янка.
Звітність про побічні ефекти
Якщо ви відчуваєте будь-які побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не перелічені в цьому описі. Також ви можете повідомити про них безпосередньо через систему фармакологічного нагляду за лікарськими засобами для людини: https://www.notificaram.es. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти забезпечити більше інформації про безпеку цього лікарського засобу.
5. Збереження Торенсу
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на упаковці після CAD. Термін придатності - останній день місяця, який вказано.
Не зберігайте при температурі вище 30 °C.
Зберігайте в оригінальній упаковці, щоб захистити від світла.
Не холодильте чи заморожуйте.
Після першого відкриття упаковки продукт може бути зберіганий протягом максимум 6 місяців.
Не використовуйте цей лікарський засіб, якщо ви спостерігаєте будь-які видимі ознаки псування, такі як замутнення розчину
Лікарські засоби не повинні викидатися у водопровідні труби чи сміття. Відкладайте упаковки та лікарські засоби, які вам не потрібні, у спеціальному місці для збору в аптеці. У разі сумнівів проконсультуйтеся з вашим фармацевтом, як позбутися упаковок та лікарських засобів, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Торенсу
- Активний інгредієнт - колекальциферол (вітамін Д3). 1 мл оральної розчини містить 10 000 МО колекальциферолу (вітамін Д3), що еквівалентно 0,25 мг. 1 крапля містить 200 МО колекальциферолу (вітамін Д3).
- Інші компоненти - оливкова олія.
Вигляд Торенсу та зміст упаковки
Торенс 10 000 МО/мл краплі оральної розчини - прозора оліозна розчина, без кольору або з жовто-зеленим кольором, без видимих твердих частинок та/або осаду. Він випускається у:
- Флаконі з окремим дозувальним піпетом
Флакон з темно-коричневого скла об'ємом 20 мл з дитячою кришкою.
Кожна упаковка містить флакон з 10 мл розчину (що відповідає 500 краплям) та дозувальний піпет з маркуванням CE 0068.
- Флаконі з дозувальним піпетом
Флакон об'ємом 20 мл з дозувальним піпетом та дитячою кришкою.
Кожна упаковка містить дозувальний піпет для видачі 10 мл розчину (що відповідає 500 краплям).
Можливо, не всі форми випуску будуть доступні на ринку
Власник дозволу на маркетинг та відповідальна особа за виробництво
Власник дозволу на маркетинг
Лабораторії Еффік, С.А.
вул. Сан-Рафаель, 3
28108 Алькобендас, Мадрид
Відповідальна особа за виробництво
Абіоген Фарма С.п.А.
вул. Меуччі, 36
Піза (Італія)
Цей лікарський засіб дозволений до застосування в країнах-членах Європейського економічного простору під наступними назвами:
Іспанія: ТОРЕНС 10 000 МО/мл краплі оральної розчини
Велика Британія: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Ірландія: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Бельгія: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Німеччина: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Нідерланди: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Норвегія: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Польща: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Португалія: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Швеція: ТОРЕНС 10 000 МО/мл оральні краплі, розчин
Дата останнього перегляду цього опису:Квітень 2021
Детальна інформація про цей лікарський засіб доступна на сайті Іспанського агентства з лікарських засобів і медичних продуктів (AEMPS) (http://www.aemps.gob.es/).

Скільки коштує ТОРЕНС 10 000 МО/мл оральні краплі в розчині в Іспанії у 2025 році?
ТОРЕНС 10 000 МО/мл оральні краплі в розчині коштує в середньому 9.37 євро у грудень, 2025 році. Ціна може змінюватися залежно від регіону, аптеки та наявності рецепта. Рекомендуємо перевіряти актуальну вартість у місцевих аптеках або через онлайн-сервіси.
- Країна реєстрації
- Середня ціна в аптеках9.37 EUR
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ТОРЕНС 10 000 МО/мл оральні краплі в розчиніФорма випуску: КАПСУЛА, 25 000 МОДіючі речовини: colecalciferolВиробник: Laboratorios Alter S.A.Потрібен рецептФорма випуску: КАПСУЛА, 50 000 МОДіючі речовини: colecalciferolВиробник: Laboratorios Alter S.A.Потрібен рецептФорма випуску: КАПСУЛА, 50 000 МОДіючі речовини: colecalciferolВиробник: Consilient Health LimitedПотрібен рецепт
Аналоги ТОРЕНС 10 000 МО/мл оральні краплі в розчині в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ТОРЕНС 10 000 МО/мл оральні краплі в розчині у Польща
Аналог ТОРЕНС 10 000 МО/мл оральні краплі в розчині у Україна
Лікарі онлайн щодо ТОРЕНС 10 000 МО/мл оральні краплі в розчині
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ТОРЕНС 10 000 МО/мл оральні краплі в розчині – за рішенням лікаря та згідно з місцевими правилами.