
ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ

Запитайте лікаря про рецепт на ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ

Інструкція із застосування ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ
Вступ
Опис: інформація для користувача
оланзапін флас синфа 5 мг буко-дисперсивні таблеткиЕФГ
Прочитайте уважно весь опис перед тим, як почати приймати цей препарат, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей препарат призначений тільки для вас і не слід давати його іншим людям, навіть якщо вони мають такі самі симптоми, як у вас, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке оланзапін флас синфа і для чого він використовується
- Що потрібно знати перед тим, як почати приймати оланзапін флас синфа
- Як приймати оланзапін флас синфа
- Можливі побічні ефекти
- Збереження оланзапіна флас синфа
- Зміст упаковки та додаткова інформація
1. Що таке оланзапін флас синфа і для чого він використовується
Оланзапін флас синфа містить активну речовину оланзапін. Оланзапін флас синфа належить до групи терапевтичних засобів антипсихотиків.
Оланзапін флас синфа призначений для лікування наступних захворювань:
- Схизофренія, захворювання, чиї симптоми є чуття, бачення або відчуття нереальних речей, помилкові переконання, підозрілість та відхід у себе. Люди, які страждають цими захворюваннями, можуть також відчувати депресію, тривогу або напруженість.
- Маніакальний розлад середньої чи важкої ступеня, захворювання, чиї симптоми є збудження або ейфорія.
Оланзапін продемонстрував свою ефективність у профілактиці повторення цих симптомів у пацієнтів з біполярним розладом, чиї маніакальні епізоди відповіли на лікування оланзапіном.
2. Що потрібно знати перед тим, як почати приймати оланзапін флас синфа
Не приймайте оланзапін флас синфа
- Якщо ви алергічні на оланзапін або на якийсь інший компонент цього препарату (включно з розділом 6). Алергічна реакція може проявлятися у вигляді висипу, свербіння, набухання обличчя або губ чи труднощів з диханням. Якщо це трапиться з вами, повідомте про це вашому лікареві.
- Якщо раніше у вас діагностували проблеми з очима, такі як певні типи глаукоми (збільшення тиску в оці).
Попередження та обережність
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як почати приймати оланзапін флас синфа
- Не рекомендується використання оланзапіна флас у пацієнтів похилого віку з деменцією, оскільки це може мати серйозні побічні ефекти.
- Препарати цього типу можуть викликати незвичайні рухи, особливо на обличчі або язиці. Якщо це трапиться з вами після прийому оланзапіна флас, повідомте про це вашому лікареві.
- У дуже рідкісних випадках препарати цього типу можуть викликати комбінацію лихоманки, прискореного дихання, потовиділення, м'язової ригідності та стану сплутаності або сонливості. Якщо це трапиться з вами, негайно зверніться до вашого лікаря.
- Було спостережено збільшення ваги у пацієнтів, які приймають оланзапін флас. Ви та ваш лікар повинні регулярно перевіряти вашу вагу. Якщо це необхідно, ваш лікар може допомогти вам спланувати дієту або розглянути можливість направлення до дієтолога.
- Було спостережено підвищення рівня цукру та жирів (тригліцеридів та холестерину) у крові у пацієнтів, які приймають оланзапін флас. Ваш лікар повинен проводити аналіз крові для контролю рівня цукру у крові та рівня жирів перед тим, як ви почнете приймати оланзапін флас, та регулярно під час лікування.
- Якщо ви або хтось з вашої сім'ї має історію тромбозу, проконсультуйтеся з вашим лікарем, оскільки препарати цього типу були асоційовані з утворенням тромбів у крові.
Якщо ви страждаєте будь-яким з наступних захворювань, повідомте про це вашому лікареві якомога скоріше:
- Інсульт чи тимчасова недостатність кровотоку в мозку (транзиторна церебральна ішемія).
- Хвороба Паркінсона.
- Проблеми з простатою.
- Блокада кишечника (паралітичний ілеус).
- Хвороба печінки чи нирок.
- Зміни в крові.
- Хвороби серця.
- Цукровий діабет.
- Конвульсії.
- Якщо ви вважаєте, що можете мати втрату солі через тривалу діарею чи блювання або внаслідок прийому діуретиків (таблеток для сечовидільної системи).
Якщо у вас діагностована деменція, ви або ваш опікун чи родич повинні повідомити про це вашому лікареві, якщо у вас раніше був інсульт чи тимчасова недостатність кровотоку в мозку.
Як правило, якщо вам більше 65 років, ваш лікар повинен регулярно перевіряти ваш тиск.
Діти та підлітки
Пацієнти молодші 18 років не повинні приймати оланзапін флас.
Прийом оланзапіна флас синфа з іншими препаратами
Використовуйте інші препарати лише за згодою вашого лікаря. Ви можете відчувати сонливість, якщо поєднаєте оланзапін флас з антидепресантами чи препаратами проти тривоги або засипання (транквілізаторами).
Повідомте вашому лікареві, якщо ви приймаєте, нещодавно приймали чи можете приймати інші препарати.
Зокрема, повідомте вашому лікареві, якщо ви приймаєте:
- Препарати для лікування хвороби Паркінсона
- Карбамазепін (антиепілептичний препарат та стабілізатор настрою), флювоксамін (антидепресант) чи ципрофлоксацин (антибіотик). Можливо, потрібно буде змінити дозу оланзапіна флас.
Прийом оланзапіна флас синфа з алкоголем
Не слід приймати алкоголь під час прийому оланзапіна флас, оскільки поєднання оланзапіна флас з алкоголем може викликати сонливість.
Вагітність та годування грудьми
Якщо ви вагітні або годуєте грудьми, вважаєте, що можете бути вагітною чи плануєте завагітніти, проконсультуйтеся з вашим лікарем перед тим, як приймати цей препарат.
Не слід приймати цей препарат під час годування грудьми, оскільки невеликі кількості оланзапіна флас можуть потрапляти до грудного молока.
Можуть виникнути наступні симптоми у новонароджених дітей, чиї матері приймали оланзапін флас у третьому триместрі вагітності (останні три місяці вагітності): тремор, м'язова ригідність та/або слабкість, сонливість, агітація, проблеми з диханням та труднощі з годуванням. Якщо ваша дитина проявляє будь-який з цих симптомів, негайно зверніться до вашого лікаря.
Водіння автомобіля та використання машин
Оланзапін може викликати симптоми, такі як сонливість, головокружіння чи зміни в зорі, та знижувати реакцію. Ці ефекти, а також сама хвороба, можуть ускладнювати вашу можливість водіння автомобіля чи використання машин. Тому не водьте, не використовуйте машини та не займаєтеся іншими діяльностями, які вимагають особливої уваги, доки ваш лікар не оцінить вашу реакцію на цей препарат.
оланзапін флас синфа містить лактозу.
Якщо ваш лікар сказав вам, що у вас є непереносимість певних цукрів, проконсультуйтеся з ним перед тим, як приймати цей препарат.
оланзапін флас синфа містить аспартам.
Цей препарат містить 0,53 мг аспартаму в кожній таблетці.
Аспартам містить джерело фенілаланіну, яке може бути шкідливим у разі фенілкетонурії (ФКН), рідкої генетичної хвороби, при якій фенілаланін накопичується через те, що організм не може правильно його вивести.
3. Як приймати оланзапін флас синфа
Слідуйте точно інструкціям щодо прийому цього препарату, вказаним вашим лікарем або фармацевтом. У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом знову.
Ваш лікар призначить вам кількість таблеток оланзапіна флас, які потрібно приймати, та тривалість лікування. Добова доза оланзапіна флас коливається від 5 мг до 20 мг. Проконсультуйтеся з вашим лікарем, якщо ви знову відчуваєте симптоми, але не припиняйте приймати оланзапін флас, якщо ваш лікар не скаже вам про це.
Таблетки оланзапіна флас потрібно приймати один раз на добу, слідуючи інструкціям вашого лікаря. Спробуйте приймати таблетки о同じ час кожної доби. Ви можете приймати їх з їжею чи без.
Буко-дисперсивні таблетки оланзапіна флас синфа призначені для перорального прийому.
Буко-дисперсивні таблетки оланзапіна флас синфа легко розчиняються, тому з ними потрібно поводитися обережно. Не торкайтеся таблеток мокрими руками, оскільки вони можуть розчинитися.
Прийом буко-дисперсивної таблетки наступний:
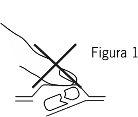
- Не розчавлюйте буко-дисперсивну таблетку
Щоб уникнути розчавлювання буко-дисперсивної таблетки, не тисніть на алвеол (Фігура 1).
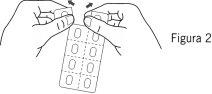
- Відокремте алвеол
Кожен блистер містить сім алвеолів, які розділені перфораціями. Відокремте алвеол, слідуючи перфорованим лініям (Фігура 2).
- Відокремте плівку
Відокремте плівку обережно, починаючи з куточка, де вказано «відокремити алюміній» (Фігури 3 та 4).
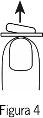
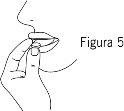
- Видаліть буко-дисперсивну таблетку
Видаліть буко-дисперсивну таблетку сухими руками та покладіть її на вашу мову (Фігура 5). Вона розчиниться безпосередньо у роті, тому буде легко її ковтати.
Також можна покласти таблетку у чашку чи склянку з водою, соком апельсина, соком яблука, молоком чи кавою, перемішавши. З деякими напоями суміш може змінити колір та стати мутною. Її потрібно випити негайно
Якщо ви прийняли більше оланзапіна флас синфа, ніж потрібно
Пацієнти, які прийняли більше оланзапіна флас, ніж потрібно, відчували наступні симптоми: прискорене серцебиття, агітація/агресивність, проблеми з мовленням, незвичайні рухи (особливо на обличчі та язиці) та зниження рівня свідомості.
Інші симптоми можуть бути: гостра сплутаність, конвульсії (епілепсія), кома, комбінація лихоманки, прискореного дихання, потовиділення, м'язової ригідності та стану сплутаності або сонливості, сповільнення частоти дихання, аспірація, підвищення артеріального тиску чи зниження артеріального тиску, аномальні ритми серця.
Зверніться до вашого лікаря або негайно зверніться до лікарні, якщо ви відчуваєте будь-який з цих симптомів. Покажіть лікареві упаковку з таблетками.
У разі передозування чи випадкового прийому проконсультуйтеся з вашим лікарем або фармацевтом чи зверніться до Токсикологічної служби, телефон: 91 562 04 20, вказавши назву препарату та кількість, прийняту.
Якщо ви забули прийняти оланзапін флас синфа
Прийоміть свою таблетку якомога скоріше, коли ви про це вспомните. Не приймайте подвійну дозу для компенсації пропущених доз.
Якщо ви припините лікування оланзапін флас синфа
Не припиняйте лікування лише тому, що ви відчуваєте себе краще. Дуже важливо продовжувати приймати оланзапін флас, доки ваш лікар не скаже вам про це.
Якщо ви припините приймати оланзапін флас раптово, можуть виникнути симптоми, такі як потовиділення, безсоння, тремор, агітація чи нудота та блювання. Ваш лікар може порекомендувати вам поступово знижувати дозу перед припиненням лікування.
Якщо у вас є якісь інші питання щодо використання цього препарату, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі препарати, цей препарат може викликати побічні ефекти, хоча не всі люди їх відчувають.
Зверніться до вашого лікаря негайно, якщо ви відчуваєте:
- Незвичайні рухи (поширений побічний ефект, який може виникнути у до 1 з 10 людей), особливо на обличчі чи язиці.
- Тромби у венах (рідкий побічний ефект, який може виникнути у до 1 з 100 людей), особливо у ногах (симптоми включають набухання, біль та почервоніння ноги), які можуть рухатися через кров до легень, викликаючи біль у грудях та труднощі з диханням. Якщо ви відчуваєте будь-який з цих симптомів, негайно зверніться до лікаря.
- Комбінація лихоманки, прискореного дихання, потовиділення, м'язової ригідності та стану сплутаності або сонливості (частота невідомачастота не може бути оцінена з наявних даних).
Дуже поширені побічні ефекти(можуть виникнути у більше 1 з 10 людей) включають збільшення ваги; сонливість; та підвищення рівня пролактину у крові. На початку лікування деякі люди можуть відчувати головокружіння чи оmdlіння (з повільнішим серцебиттям), особливо при встанні з положення лежачи чи сидячого. Це відчуття зазвичай проходить самостійно, але якщо це не трапиться, проконсультуйтеся з вашим лікарем.
Поширені побічні ефекти(можуть виникнути у до 1 з 10 людей) включають зміни в рівнях деяких клітин крові, ліпідів у крові та на початку лікування тимчасові підвищення рівня печінкових ферментів; підвищення рівня цукру у крові та сечі; підвищення рівня сечовини та креатинфосфокінази у крові; підвищення апетиту; головокружіння; агітація; тремор; незвичайні рухи (дискінезія); запор; сухість у роті; висип; втрата сили; надмірна втома; затримання рідини, яке викликає набухання рук, щиколоток чи ніг; лихоманка, біль у суглобах та сексуальні дисфункції, такі як зниження лібідо у чоловіків та жінок чи еректильна дисфункція у чоловіків.
Рідкі побічні ефекти(можуть виникнути у до 1 з 100 людей) включають надмірне слиновиділення, гіперчутливість (напр., запалення рота та горла, свербіння, висип); цукровий діабет чи погіршення діабету, пов'язані з кетоацидозом (ацетон у крові та сечі) чи комою; конвульсії, у більшості випадків пов'язані з попередніми конвульсіями (епілепсією); м'язова ригідність чи спазми (включно з рухами очей); синдром неспокійних ніг; проблеми з мовленням; заїкання; повільне серцебиття; чутливість до сонячного світла; носові кровотечі;腹ова distension; втрата пам'яті чи забування; недержання сечі; втрата здатності сечовидільної системи; втрата волосся; відсутність чи зниження менструації; та зміни в молочній залозі у чоловіків та жінок, такі як аномальне виділення молока чи аномальний ріст.
Дуже рідкі побічні ефекти(можуть виникнути у до 1 з 1 000 людей) включають зниження температури тіла; аномальний ритм серця; раптова смерть без очевидної причини; запалення підшлункової залози, яке викликає сильний біль у животі, лихоманку та нездужання; захворювання печінки, яке викликає жовтушність шкіри та білків очей; м'язовий розлад, який проявляється у вигляді болю без очевидної причини та тривалої чи болючої ерекції.
Дуже рідкі побічні ефекти(можуть виникнути у до 1 з 10 000 людей) включають серйозні алергічні реакції, такі як реакція на препарат з еозінофілією та системними симптомами (DRESS за англійською абревіатурою). Спочатку DRESS проявляється симптомами, подібними до грипу з висипом на обличчі, а потім з поширеним висипом, лихоманкою, збільшенням лімфатичних вузлів, підвищенням рівня печінкових ферментів у крові та підвищенням рівня одного типу білих клітин крові (еозінофілів).
Під час лікування оланзапіном пацієнти похилого віку з деменцією можуть відчувати інсульт, пневмонію, недержання сечі, падіння, надмірну втому, візуальні галюцинації, підвищення температури тіла, почервоніння шкіри та проблеми з ходьбою. Було зареєстровано деякі випадки смерті у цій конкретній групі пацієнтів.
Оланзапін флас може погіршувати симптоми у пацієнтів з хворобою Паркінсона.
Звіт про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не вказані в цьому описі. Ви також можете повідомити про них безпосередньо через Систему моніторингу безпеки лікарських засобів для людини: https://www.notificaram.es. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього препарату.
5. Збереження оланзапіна флас синфа
Тримайте цей препарат поза зоною досяжності дітей.
Не використовуйте цей препарат після закінчення терміну придатності, вказаного на упаковці після CAD. Термін придатності закінчується в останній день місяця, вказаного.
Цьому препарату не потрібні спеціальні умови зберігання.
Лікарські засоби не повинні викидатися у водопровід чи сміття. Відкладайте упаковки та лікарські засоби, які вам не потрібні, у пункті збору(SIGRE) аптеки. У разі сумнівів проконсультуйтеся з вашим фармацевтом, як позбутися упаковок та лікарських засобів, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад оланзапіну флас синфа
- Активна речовина - оланзапін. Кожна буккальна таблетка оланзапіну флас синфа містить 5 мг активної речовини.
- Інші компоненти: лактоза моногідрат, силікат кальцію, гідроксипропілцелюлоза низької заміни, кросповідон, аспартам, аромат апельсина, аромат банана, колоїдна безводна діоксид силіцію та стеарат магнію.
Вигляд продукту та вміст упаковки
Оланзапін флас синфа 5 мг буккальні таблетки - це круглі та жовті таблетки.
Оланзапін флас синфа 5 мг буккальні таблетки випускаються в упаковках по 28 таблеток.
Інші форми випуску:
Оланзапін флас синфа 10 мг буккальні таблетки: упаковки по 28 і 56 таблеток.
Власник дозволу на торгівлюта відповідальний за виробництво
Власник дозволу на торгівлю
Лабораторії Сінфа, С.А.
Карретера Олаз-Чіпі, 10. Полігональна промисловість Арета
31620 Уарте (Наварра) - Іспанія
Відповідальний за виробництво
Лабораторії Сінфа, С.А.
Карретера Олаз-Чіпі, 10. Полігональна промисловість Арета
31620 Уарте (Наварра) - Іспанія
або
Нейраксфарм Фармацевтика, С.Л.
Авда де Барселона, 69
Сант Жоан Деспі (Барселона)
Іспанія
Дата останнього перегляду цього проспекту:Квітень 2020
Детальна та оновлена інформація про цей лікарський засіб доступна на сайті Іспанського агентства лікарських засобів та медичних продуктів (AEMPS) http://www.aemps.gob.es/.
Ви можете отримати детальну та оновлену інформацію про цей лікарський засіб, скануючи з вашого мобільного телефону (смартфона) код QR, включений до проспекту та картонажу. Також ви можете отримати цю інформацію на наступному сайті інтернету: https://cima.aemps.es/cima/dochtml/p/73686/P_73686.html
Код QR на: https://cima.aemps.es/cima/dochtml/p/73686/P_73686.html

Скільки коштує ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ в Іспанії у 2025 році?
ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ коштує в середньому 26.44 євро у грудень, 2025 році. Ціна може змінюватися залежно від регіону, аптеки та наявності рецепта. Рекомендуємо перевіряти актуальну вартість у місцевих аптеках або через онлайн-сервіси.
- Країна реєстрації
- Середня ціна в аптеках26.44 EUR
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯФорма випуску: ТАБЛЕТКА, 10 мгДіючі речовини: olanzapineВиробник: Neuraxpharm Spain S.L.Потрібен рецептФорма випуску: ТАБЛЕТКА, 2,5 мгДіючі речовини: olanzapineВиробник: Neuraxpharm Spain S.L.Потрібен рецептФорма випуску: ТАБЛЕТКА, 5 мгДіючі речовини: olanzapineВиробник: Neuraxpharm Spain S.L.Потрібен рецепт
Аналоги ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ у Польща
Аналог ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ у Україна
Лікарі онлайн щодо ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ОЛАНЗАПІН ФЛАС ЦИНФА 5 мг ТАБЛЕТКИ ДЛЯ ПЕРОРАЛЬНОГО РОЗЧИНЕННЯ – за рішенням лікаря та згідно з місцевими правилами.









