

ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ

Запитайте лікаря про рецепт на ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ

Інструкція із застосування ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ
Введення
Опис: інформація для користувача
Фульвестрант Доктор Реддіс 250 мг розчин для ін'єкційу попередньо заповненому шприці EFG
Перш ніж почати використовувати цей лікарський засіб, уважно прочитайте весь опис, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас виникли питання, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
- Цей лікарський засіб призначений лише вам, і не давайте його іншим людям, навіть якщо вони мають такі самі симптоми, як і ви, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке Фульвестрант Доктор Реддіс і для чого він використовується
- Що вам потрібно знати перед початком використання Фульвестранту Доктор Реддіс
- Як використовувати Фульвестрант Доктор Реддіс
- Можливі побічні ефекти
- Збереження Фульвестранту Доктор Реддіс
- Зміст упаковки та додаткова інформація
1. Що таке Фульвестрант Доктор Реддіс і для чого він використовується
Фульвестрант Доктор Реддіс містить активну речовину фульвестрант, яка належить до групи блокаторів естрогену. Естрогени, тип жіночих статевих гормонів, можуть бути причетні до розвитку деяких видів раку молочної залози.
Фульвестрант використовується:
- У вигляді монотерапії для лікування жінок після менопаузи з певним типом раку молочної залози, який називається раком молочної залози з позитивним рецептором естрогену, який є локально розширеним або поширеним на інші частини тіла (метастатичним) або,
- у поєднанні з палбоциблібом для лікування жінок з певним типом раку молочної залози, який називається раком молочної залози з позитивним гормональним рецептором, раком молочної залози з негативним рецептором 2 людського епідермального фактору росту, який є локально розширеним або поширеним на інші частини тіла (метастатичним). Жінки, які не досягли менопаузи, також будуть лікуватися лікарським засобом, який називається агоністом гормону, що виділяє лютеїнізуючий гормон (ЛГРГ).
Фульвестрант може бути призначений у поєднанні з палбоциблібом. Важливо, щоб ви також прочитали опис палбоциблібу. Якщо у вас виникли питання щодо палбоциблібу, проконсультуйтеся з вашим лікарем.
2. Що вам потрібно знати перед початком використання Фульвестранту Доктор Реддіс
Не використовуйте Фульвестрант Доктор Реддіс:
- якщо ви алергічні на фульвестрант або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6)
- якщо ви вагітні або годуєте грудьми
- якщо у вас є тяжкі порушення функції печінки
Попередження та обережність
Проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою перед початком використання Фульвестранту Доктор Реддіс, якщо будь-який з наступних пунктів стосується вас:
- проблеми з нирками або печінкою
- низький рівень тромбоцитів (які допомагають згортанню крові) або порушення згортання крові
- попередні проблеми з тромбозом
- остеопороз (втрата густини кісткової тканини)
- алкоголізм
Діти та підлітки
Фульвестрант не призначений для дітей та підлітків молодше 18 років.
Використання Фульвестранту Доктор Реддіс з іншими лікарськими засобами
Повідомте вашого лікаря або фармацевта, якщо ви приймаєте, нещодавно приймали або можете приймати будь-який інший лікарський засіб.
Зокрема, ви повинні повідомити вашому лікареві, якщо ви використовуєте антикоагулянти (лікарські засоби для профілактики тромбозу).
Вагітність та годування грудьми
Не використовуйте фульвестрант, якщо ви вагітні. Якщо ви можете завагітніти, ви повинні використовувати ефективний метод контрацепції під час лікування фульвестрантом та протягом 2 років після останньої дози.
Не годуйте грудьми під час лікування фульвестрантом.
Водіння автомобіля та використання машин
Не очікується, що фульвестрант буде впливати на вашу здатність водити автомобіль або використовувати машини. Однак, якщо ви відчуваєте втому після лікування, не водьте автомобіль та не використовуйте машини.
Етанол
Цей лікарський засіб містить 500 мг алкоголю (етанолу) на 5 мл розчину. Кількість у одній дозі (два шприці по 5 мл) цього лікарського засобу еквівалентна менш ніж 25 мл пива або 10 мл вина. Не ймовірно, що кількість алкоголю в цьому лікарському засобі матиме ефект на дорослих та підлітків. Алкоголь у цьому лікарському засобі може змінити ефекти інших лікарських засобів. Проконсультуйтеся з вашим лікарем або фармацевтом, якщо ви приймаєте інші лікарські засоби. Якщо ви алкоголік, проконсультуйтеся з вашим лікарем або фармацевтом перед прийняттям цього лікарського засобу.
Бензиловий спирт
Цей лікарський засіб містить 500 мг бензилового спирту в кожному флаконі по 5 мл. Бензиловий спирт може викликати алергічні реакції.
Проконсультуйтеся з вашим лікарем або фармацевтом, якщо у вас є захворювання печінки або нирок. Це пов'язано з тим, що великі кількості бензилового спирту можуть накопичуватися в вашому організмі та викликати побічні ефекти (що називається "метаболічною ацидозою").
Бензоат бензилу
Цей лікарський засіб містить 750 мг бензоату бензилу в кожному флаконі по 5 мл.
Рицинову олію
Може викликати сильні алергічні реакції.
3. Як використовувати Фульвестрант Доктор Реддіс
Слідуйте точно інструкціям з адміністрації цього лікарського засобу, вказаним вашим лікарем або фармацевтом. У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом.
Рекомендована доза становить 500 мг фульвестранту (дві ін'єкції по 250 мг)一次 на місяць з додатковою дозою 500 мг, яку вводять через 2 тижні після початкової дози.
Ваш лікар або медсестра введуть фульвестрант шляхом повільної інтрамускульної ін'єкції в кожен з ваших сідниць.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Вам може знадобитися термінове медичне лікування, якщо ви відчуваєте будь-який з наступних побічних ефектів:
- Алергічні реакції (гіперчутливість), включаючи набряк обличчя, губ, язика та/або горла, які можуть бути ознаками анафілактичних реакцій
- Тромбоемболія (збільшення ризику тромбозу)*
- Запалення печінки (гепатит)
- Порушення функції печінки
Негайно повідомте вашому лікареві, фармацевту або медсестрі, якщо ви відчуваєте будь-який з наступних побічних ефектів:
Дуже часті побічні ефекти(можуть впливати на більш ніж 1 з 10 людей)
- Реакції в місці ін'єкції, такі як біль та/або запалення
- Аномальні рівні ферментів печінки (у аналізі крові)*
- Нудота (чуття нездоров'я)
- Слабкість, втома*
- Біль у суглобах та м'язах
- Потіння
- Висип
- Алергічні реакції (гіперчутливість), які включають набряк обличчя, губ, язика та/або горла
Всі інші побічні ефекти:
Часті побічні ефекти(можуть впливати на до 1 з 10 людей)
- Головний біль
- Воміта, діарея або втрата апетиту*
- Інфекції сечовидільної системи
- Біль у спині*
- Збільшення рівня білірубіну (пігменту жовчі, який виробляється печінкою)
- Тромбоемболія (збільшення ризику тромбозу)*
- Зниження рівня тромбоцитів (тромбоцитопенія)
- Вагінальне кровотеча
- Біль у попереку, який віддає в ногу (ішіас)
- Раптова слабкість, оніміння, поколювання або втрата руху в нозі, особливо на одному боці тіла, раптові проблеми з ходьбою або балансом (периферична нейропатія)
Побічні ефекти, які спостерігаються рідше(можуть впливати на до 1 з 100 людей)
- Густий, білуватий вагінальний секрет та кандидоз (інфекція)
- Гематома та кровотеча в місці ін'єкції
- Збільшення рівня гамма-ГТ, ферменту печінки, який визначається в аналізі крові
- Запалення печінки (гепатит)
- Порушення функції печінки
- Оніміння, поколювання та біль
- Анафілактичні реакції
- Включає побічні ефекти, для яких не можна точно оцінити роль фульвестранту через основне захворювання.
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, повідомте вашому лікареві, фармацевту або медсестрі, навіть якщо це побічні ефекти, які не вказані в цьому описі. Ви також можете повідомити про них безпосередньо через Систему моніторингу лікарських засобів для людини: http://www.notificaram.es. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Збереження Фульвестранту Доктор Реддіс
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на упаковці або на етикетці шприця після "CAD".
Термін придатності - останній день місяця, який вказано.
Цьому лікарському засобу не потрібні спеціальні умови зберігання при певній температурі.
Зберігайте шприц у первинній упаковці, щоб захистити його від світла.
Ваш лікар буде відповідальний за правильне зберігання, використання та утилізацію фульвестранту.
Цей лікарський засіб може становити ризик для водної екосистеми. Лікарські засоби не повинні викидати в каналізацію чи сміття. Відкладайте упаковку та лікарські засоби, які вам не потрібні, в пункті збору SIGRE аптеки. Спитайте вашого фармацевта, як утилізувати упаковку та лікарські засоби, які вам не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Фульвестранту Доктор Реддіс
- Активна речовина - фульвестрант. Кожен шприц (5 мл) містить 250 мг фульвестранту.
Кожен мл містить 50 мг фульвестранту.
- Інші компоненти (експіцієнти) - етанол (96%), бензиловий спирт, бензоат бензилу та рицинова олія.
Вигляд продукту та зміст упаковки
Фульвестрант Доктор Реддіс - це в'язка, прозора, безбарвна або жовтувата рідина в шприці, який оснащений захистом від відкриття, що містить 5 мл ін'єкційного розчину. Для отримання рекомендованої місячної дози 500 мг потрібно вводити дві шприци.
Фульвестрант Доктор Реддіс випускається у 4 формах: упаковці, яка містить 1 шприц, упаковці, яка містить 2 шприци, упаковці, яка містить 4 шприци, або упаковці, яка містить 6 шприців. До кожного шприця додаються голки з системою безпеки (Terumo® SurGuard® 3) для підключення до тіла та руків'я.
Можливо, що тільки деякі розміри упаковок будуть випускатися.
Власник дозволу на маркетинг та відповідальна особа за виробництво
Власник дозволу на маркетинг:
Reddy Pharma Iberia, S.A.U.
Адреса: Avenida Josep Tarradellas, nº 38
08029 Барселона (Іспанія)
Телефон: +34 93 355 49 16
Факс: +34 93 355 49 61
Відповідальна особа за виробництво:
Betapharm Arzneimittel GmbH
Адреса: Kobelweg 95-Augsburg
86156
Німеччина
Номер факсу: +004982174881-420
Номер телефону: +004982174881-0
Або
S.C. Rual Laboratories S.R.L.,
Адреса: 313, Splaiul Unirii, Building H, 1st floor,
сектор 3, Бухарест,
030138, Румунія
Цей лікарський засіб дозволений в державах-членах Європейського економічного простору під наступними назвами:
Німеччина | Фульвестрант бета 250мг/5мл ін'єкційний розчин у попередньо заповненому шприці |
Іспанія | Фульвестрант Доктор Реддіс 250 мг розчин для ін'єкцій у попередньо заповненому шприці EFG |
Франція | Фульвестрант Доктор Реддіс 250 мг, розчин для ін'єкцій у попередньо заповненому шприці |
Італія | Фульвестрант Доктор Реддіс 250 мг/5 мл ін'єкційний розчин |
Румунія | Фалвакс Доктор Реддіс 250 мг/5 мл, ін'єкційний розчин у попередньо заповненому шприці |
Велика Британія | Фульвестрант Доктор Реддіс 250 мг/5 мл розчин для ін'єкцій у попередньо заповненому шприці |
Дата останнього перегляду
----------------------------------------------------------------------------------------------------------------------
Ця інформація призначена лише для медичних працівників:
Фульвестрант Доктор Реддіс 500 мг (дві ін'єкції по 250 мг) повинен вводитися за допомогою двох попередньо заповнених шприців, див. розділ 3.
Інструкції з адміністрації
Попередження - не стерилізуйте голку з системою безпеки (Terumo® SurGuard® 3) перед її використанням. Руки повинні залишатися позаду голки під час її використання та утилізації.
Для кожної з двох шприців:
Фігура 1
- Видаліть скляний корпус шприця з упаковки та перевірте, чи не пошкоджений він.
- Зламайте пломбу прозорого пластикового захисту конектора Luer-Lock шприця, щоб видалити цей захист з гумовою кришкою на кінчику (див. Фігура 1).
Фігура 2
- Відкрийте зовнішню упаковку голки з системою безпеки. Підключіть голку з системою безпеки до Luer-Lock (див. Фігура 2).
- Поверніть до тих пір, поки не закріпиться та не заблокується міцно на конекторі.
- Ведіть шприц до місця ін'єкції.
Фігура 3
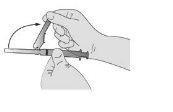 Перш ніж видалити кришку голки, видаліть захисний кожух у напрямку до циліндра шприця під кутом, який показано.
Перш ніж видалити кришку голки, видаліть захисний кожух у напрямку до циліндра шприця під кутом, який показано.
- Перш ніж зробити ін'єкцію, візуально перевірте парентеральні розчини на наявність частинок та забарвлення.
- Видаліть надлишковий газ з шприця.
- Введіть повільно через інтрамускульну ін'єкцію в сідницю (1-2 хвилини/ін'єкція). Для більшої зручності, положення голки зрізом вгору має ту саму орієнтацію, що й руків'я, підняте вгору (див. Фігура 3).
- Використайте руків'я, якщо потрібно
Фігура 4
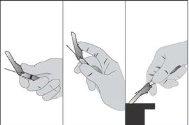 Після ін'єкції, щоб активувати механізм безпеки, виконайте техніку однією рукою, використовуючи будь-який з трьох методів, показаних раніше (активація підтверджується чутним або тактильним "кліком" та може бути візуально підтверджена) (див. Фігура 4).
Після ін'єкції, щоб активувати механізм безпеки, виконайте техніку однією рукою, використовуючи будь-який з трьох методів, показаних раніше (активація підтверджується чутним або тактильним "кліком" та може бути візуально підтверджена) (див. Фігура 4).
ПРИМІТКА: Активуйте на відстані від вашого тіла та інших людей. Почуйте клік та візуально підтвердьте, що голка повністю захищена.
Утилізація
Попередньо заповнені шприци призначені тількидля одного використання.
Цей лікарський засіб може становити ризик для водної екосистеми. Утилізація незастосованого лікарського засобу та всіх матеріалів, які були в контакті з ним, повинна здійснюватися згідно з місцевими правилами.

Скільки коштує ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ в Іспанії у 2025 році?
ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ коштує в середньому 408.86 євро у грудень, 2025 році. Ціна може змінюватися залежно від регіону, аптеки та наявності рецепта. Рекомендуємо перевіряти актуальну вартість у місцевих аптеках або через онлайн-сервіси.
- Країна реєстрації
- Середня ціна в аптеках408.86 EUR
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 250 мг/5 млДіючі речовини: fulvestrantВиробник: Bexal Farmaceutica S.A.Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 250 мгДіючі речовини: fulvestrantВиробник: Ever Valinject GmbhПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 250 мгДіючі речовини: fulvestrantВиробник: Astrazeneca AbПотрібен рецепт
Аналоги ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ у Польща
Аналог ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ у Україна
Лікарі онлайн щодо ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ФУЛВЕСТРАНТ ДР. РЕДДІС 250 мг РОЗЧИН ДЛЯ ІН'ЄКЦІЙ У ПЕРЕДНАПОВНЕНІЙ ШПРИЦІ – за рішенням лікаря та згідно з місцевими правилами.












