
FINOMEL PERI EMULSION PARA PERFUSION

Cómo usar FINOMEL PERI EMULSION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Finomel Peri emulsión para perfusión
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Finomel Peri y para qué se utiliza
- Qué necesita saber antes de que empezar a usar Finomel Peri
- Cómo usar Finomel Peri
- Posibles efectos adversos
- Conservación de Finomel Peri
6.Contenido del envase e información adicional
1. Qué es Finomel Peri y para qué se utiliza
Finomel Peri contiene aminoácidos (componentes utilizados para construir proteínas), glucosa (carbohidratos), lípidos (grasa) y sales (electrolitos).
Finomel Peri se utiliza para alimentar a adultos cuando la alimentación normal por la boca es insuficiente o no es posible.
2. Qué necesita saber antes de empezar a usar Finomel Peri
No use Finomel Peri:
- Si es alérgico a las proteínas de pescado, huevo, soja, cacahuete, al maíz/productos de maíz (ver también la sección “Advertencias y precauciones” a continuación), o a cualquiera de los componentes de este medicamento (ver la lista en la sección 6).
- Si tiene altos niveles de grasas en la sangre.
- Si tiene problemas graves con el hígado.
- Si tiene problemas de coagulación sanguínea.
- Si tiene un trastorno que impide a su cuerpo procesar los aminoácidos.
- Si tiene problemas graves con los riñones.
- Si tiene demasiado azúcar en la sangre.
- Si tiene una cantidad anormalmente elevada de cualquiera de los electrolitos (sodio, potasio, magnesio, calcio y/o fósforo) en la sangre.
- Si tiene problemas para recibir grandes volúmenes de líquidos en las venas, como edema pulmonar agudo, hiperhidratación y problemas cardiacos descompensados.
- Si tiene cualquier problemas de salud agudo y grave, como condiciones postraumáticas graves, diabetes mellitus no controlada, infarto agudo de miocardio, accidente cerebrovascular, embolia, acidosis metabólica, sepsis grave (bacterias en la sangre), deshidratación hipotónica y coma hiperosmolar.
En todos los casos, su médico decidirá si se le debe administrar este medicamento en función de factores como su edad, peso y estado clínico, junto con los resultados de todas las pruebas realizadas.
Advertencias y precauciones
Consulte a su médico o enfermero antes de empezar a usar Finomel Peri si tiene:
- algún problema grave de riñón. Debe informar también a su médico si está recibiendo tratamiento de diálisis (riñón artificial) o si tiene otro tipo de tratamiento para limpiar la sangre.
- algún problema grave de hígado
- algún problema de coagulación de la sangre
- funcionamiento anormal de las glándulas adrenales (insuficiencia adrenal). Las glándulas suprarrenales son glándulas en forma de triángulo ubicadas en la parte superior de los riñones.
- insuficiencia cardiaca
- enfermedad pulmonar
- acumulación de agua en el organismo (hiperhidratación)
- cantidad insuficiente de agua en el organismo (deshidratación)
- exceso de azúcar en la sangre (diabetes mellitus) sin recibir tratamiento para ello
- ataque al corazón o shock debido a un fallo cardíaco repentino
- acidosis metabólica grave (sangre demasiado ácida)
- infección generalizada (septicemia)
La perfusión debe detenerse inmediatamente si se desarrolla cualquier signo o síntoma anormales de una reacción alérgica, como fiebre, escalofríos, erupciones en la piel o dificultad para respirar. Este medicamento contiene aceite de pescado, aceite de soja, fosfátidos de huevo y glucosa derivada del maíz que pueden causar reacciones de hipersensibilidad. Se han observado reacciones alérgicas cruzadas entre las proteínas de la semilla de soja y del cacahuete.
La dificultad para respirar también podría ser una señal de que en los pulmones se han formado pequeñas partículas que bloquean los vasos sanguíneos (precipitados vasculares pulmonares). Si experimenta cualquier dificultad para respirar, informe a su médico o enfermero. Ellos decidirán la acción a tomar.
Durante la perfusión, si observa dolor, ardor, rigidez, hinchazón o cambio de color de la piel en el lugar de la perfusión o alguna fuga durante la perfusión,avise a su médico o enfermero. Se detendrá inmediatamente la administración y se reiniciará en otra vena.
Existe riesgo de infección o de sepsis (presencia de bacterias o sus toxinas en la sangre), especialmente cuando se coloca un tubo (catéter intravenoso) en la vena. El médico le observará atentamente en busca de signos de infección. El uso de "técnicas asépticas" (sin gérmenes) al colocar y realizar el mantenimiento del catéter y al preparar la fórmula nutricional puede reducir el riesgo de infección.
Se han descrito casos de síndrome de sobrecarga de grasa con productos similares. Una reducción o limitación de la capacidad del cuerpo para eliminar las grasas que contiene Finomel Peri puede ocasionar un síndrome de sobrecarga de grasas (ver la sección 4: Posibles efectos secundarios).
Si usted está gravemente desnutrido de forma tal que necesite recibir alimentación por vía intravenosa, se recomienda iniciar la nutrición parenteral cuidadosamente y con lentitud.
Pruebas de laboratorio adicionales
Antes de comenzar la perfusión, deberán corregirse los trastornos metabólicos y el equilibrio de agua y electrolitos de su organismo. Para comprobar la eficacia y la seguridad de la administración, su médico podrá realizarle pruebas de laboratorio y clínicas mientras se le administre este medicamento. Su médico supervisará su estado y podrá cambiar la dosis o añadirle otra medicación.
Niños y adolescentes
No hay experiencia con el uso de Finomel Peri en niños y adolescentes.
Otros medicamentos y Finomel Peri
Informe a su médico o enfermera si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Finomel Peri contiene calcio. No debe administrarse junto o a través de la misma vía con el antibiótico ceftriaxona porque podrían formarse partículas. Si se utiliza el mismo dispositivo para administrarle estos medicamentos de forma sucesiva, se debe enjuagar bien.
Los aceites de oliva y de soja presentes en Finomel Peri contienen vitamina K. Esto no suele afectar a los medicamentos para fluidificar la sangre (anticoagulantes), como la cumarina. Sin embargo, si toma anticoagulantes debe decírselo a su médico.
Los lípidos que contiene esta emulsión pueden interferir con los resultados de ciertas pruebas de laboratorio si la muestra de sangre se toma antes de que se hayan eliminado de su flujo sanguíneo (se eliminan generalmente tras un período de 5 a 6 horas sin recibir los lípidos).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de tomar este medicamento. No hay datos sobre el uso de Finomel Peri en mujeres embarazadas o en periodo de lactancia. Puede considerarse el uso de este medicamento durante el embarazo y la lactancia si su médico determina que es necesario.
Conducción y uso de máquinas
No procede, ya que el medicamento se administra en el hospital.
3. Cómo usar Finomel Peri
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico.
Este medicamento se administra por perfusión intravenosa (goteo) a través de un pequeño tubo, directamente en una vena.
Su médico decidirá la dosis que se le administrará de forma individualizada dependiendo de su peso corporal y su estado funcional. Un profesional sanitario le administrará Finomel Peri.
Uso en niños
La seguridad y eficacia en niños y adolescentes de menos de 18 años de edad no han sido establecidas.
Si usa más Finomel Peri del que debe
Es poco probable que reciba demasiado medicamento, ya que un profesional sanitario le administrará Finomel Peri.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los siguientes efectos adversos se han comunicado con una frecuencia desconocida:
- reacciones de hipersensibilidad (que pueden darle síntomas como hinchazón, fiebre, caída de la presión arterial, erupciones cutáneas, ronchas (manchas rojas elevadas), rubefacción, cefalea;
- síndrome de realimentación (una enfermedad que se desarrolla cuando recibe alimentación después de largos periodos de ayuno);
- concentraciones elevadas de azúcar en la sangre (hiperglucemia);
- mareos;
- dolor de cabeza;
- inflamación de la vena (tromboflebitis);
- embolia pulmonar;
- dificultades para respirar;
- náuseas;
- vómitos;
- leve aumento de la temperatura corporal;
- altas concentraciones en sangre (plasma) de compuestos del hígado;
- síndrome de sobrecarga de grasa;
- pérdida de la perfusión en el tejido circundante (extravasación).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Finomel Peri
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en la sobrebolsa. No congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta de la bolsa y el embalaje exterior después de CAD. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa partículas visibles en la solución o si la bolsa está dañada.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Finomel Peri
- Los principios activos son
g por 1000 ml | |
Alanina | 6,52 |
Arginina | 3,62 |
Glicina | 3,24 |
Histidina | 1,51 |
Isoleucina | 1,89 |
Leucina | 2,30 |
Lisina (equivalente a clorhidrato) | 2,28 |
Metionina | 1,26 |
Fenilalanina | 1,76 |
Prolina | 2,14 |
Serina | 1,58 |
Treonina | 1,32 |
Triptófano | 0,57 |
Tirosina | 0,13 |
Valina | 1,83 |
Acetato sódico trihidrato | 1,77 |
Cloruro potásico | 1,41 |
Cloruro cálcico dihidrato | 0,23 |
Sulfato magnésico heptahidrato | 0,78 |
Glicerofosfato sódico hidratado | 1,87 |
Sulfato de zinc heptahidrato | 0,007 |
Glucosa (equivalente a monohidrato) | 77,8 |
Aceite de soja refinado | 8,46 |
Aceite de oliva refinado | 7,05 |
Triglicéridos de cadena media | 7,05 |
Aceite de pescado rico en ácidos omega-3 | 5,64 |
- Los demás componentes son: ácido acético glacial, ácido clorhídrico, fosfolípidos de huevo, glicerol, oleato de sodio, todo-rac-α-tocoferol, hidróxido de sodio, agua para preparaciones inyectables.
Aspecto de Finomel Peri y contenido del envase
Las soluciones de aminoácidos y glucosa son trasparentes, incoloras o ligeramente amarillentas y libres de partículas. La emulsión de lípidos es homogénea y de color blanco.
Después de mezclar las 3 cámaras, el producto tiene el aspecto de una emulsión blanca.
Tamaños de envases
4 bolsas de 1085 ml
4 bolsas de 1450 ml
4 bolsas de 2020 ml
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Baxter SL
Pouet de Camilo, 2.
46394 Ribarroja del Turia (Valencia)
España
Responsable de la fabricación:
Baxter SA
Boulevard René Branquart 80
7860 Lessines
Bélgica
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Austria, República Checa, Alemania, Grecia, Irlanda, Finomel PeriPolonia, España, Reino Unido
Bélgica, Luxemburgo, Holanda Periomegomel
Dinamarca, Finlandia, Islandia, Noruega, Suecia Finomel Perifer
Francia Fosomelperi
Italia Finomel
Fecha de la última revisión de este prospecto: Diciembre 2023
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
<------------------------------------------------------------------------------------------------------------------------>
Esta información está destinada únicamente a profesionales del sector sanitario
- COMPOSICIÓN CUALITATIVA Y CUANTITATIVA
Finomel Peri se presenta en una bolsa de plástico con 3 compartimentos. Cada bolsa contiene una combinación estéril y apirógena de solución de glucosa al 13 %, una solución de aminoácidos al 10 % con electrolitos y una emulsión de lípidos al 20 %.
Tras mezclar el contenido de los 3 compartimentos, la composición de la emulsión reconstituida se indica en la siguiente tabla:
Sustancia activa | 1085 ml | 1450 ml | 2020 ml |
Aceite de pescado rico en ácidos omega-3 | 6,12 g | 8,16 g | 11,40 g |
Aceite de oliva refinado | 7,65 g | 10,20 g | 14,25 g |
Aceite de soja refinado | 9,18 g | 12,24 g | 17,10 g |
Triglicéridos de cadena media | 7,65 g | 10,20 g | 14,25 g |
Alanina | 7,08 g | 9,46 g | 13,17 g |
Arginina | 3,93 g | 5,26 g | 7,31 g |
Glicina | 3,52 g | 4,71 g | 6,55 g |
Histidina | 1,64 g | 2,19 g | 3,05 g |
Isoleucina | 2,05 g | 2,74 g | 3,82 g |
Leucina | 2,50 g | 3,34 g | 4,64 g |
Lisina(equivalente a lisina clorhidrato) | 1,98 g(2,48 g) | 2,65 g(3,31 g) | 3,69 g(4,61 g) |
Metionina | 1,37 g | 1,83 g | 2,54 g |
Fenilalanina | 1,92 g | 2,56 g | 3,56 g |
Prolina | 2,33 g | 3,11 g | 4,32 g |
Serina | 1,71 g | 2,29 g | 3,18 g |
Treonina | 1,44 g | 1,92 g | 2,67 g |
Triptófano | 0,62 g | 0,82 g | 1,14 g |
Tirosina | 0,14 g | 0,18 g | 0,25 g |
Valina | 1,98 g | 2,65 g | 3,69 g |
Acetato sódico trihidrato | 1,92 g | 2,57 g | 3,57 g |
Cloruro potásico | 1,53 g | 2,05 g | 2,85 g |
Cloruro cálcico dihidrato | 0,25 g | 0,34 g | 0,47 g |
Sulfato magnésico heptahidrato | 0,84 g | 1,13 g | 1,57 g |
Glicerofosfato sódico hidratado | 2,03 g | 2,71 g | 3,77 g |
Sulfato de zinc heptahidrato | 0,008 g | 0,011 g | 0,015 g |
Glucosa(equivalente a glucosa monohidratada) | 76,7 g(84,4 g) | 102,6 g(112,8 g) | 142,9 g(157,2 g) |
- POSOLOGÍA Y FORMA DE ADMINISTRACIÓN
Posología
La dosis debe individualizarse en función del gasto energético del paciente, su estado clínico, su peso corporal y su capacidad para metabolizar los componentes de Finomel Peri, así como de la energía o de las proteínas adicionales administradas por vía oral o enteral. Por tanto, debe elegirse el tamaño de la bolsa apropiado.
Las necesidades diarias promedio en adultos son:
- En pacientes con estado de alimentación normal o en condiciones de estrés catabólico leve: de 0,6 a 0,9 g de aminoácidos/kg peso corporal/día (de 0,10 a 0,15 g de nitrógeno/kg peso corporal/día).
- En pacientes con estrés metabólico de moderado a alto, con o sin desnutrición: de 0,9 a 1,6 g de aminoácidos/kg peso corporal/día (de 0,15 a 0,25 g de nitrógeno/kg peso corporal/día).
- En pacientes con condiciones especiales (p. ej., quemaduras o anabolismo marcado) las necesidades de nitrógeno pueden ser aún más altas.
La dosis diaria máxima varía según el estado clínico del paciente y puede cambiar de un día a otro.
La velocidad de administración debe aumentarse gradualmente durante la primera hora.
La velocidad de administración debe ajustarse teniendo en cuenta la dosis que se administra, la ingesta diaria de volumen y la duración de la perfusión.
El tiempo de perfusión recomendado es de 14 a 24 horas.
El régimen de 20 ml a 40 ml/kg peso corporal/día corresponde a 0,6-1,3 g de aminoácidos/kg peso corporal/día (correspondiente a 0,10-0,21 g de nitrógeno/kg peso corporal/día) y a 14-27 kcal/kg peso corporal/día de energía total (11-22 kcal/kg peso corporal/día de energía no proteica).
La velocidad de perfusión máxima para la glucosa es de 0,25 g/kg peso corporal/h, para aminoácidos 0,1 g/kg peso corporal/h y para lípidos 0,15 g/kg peso corporal/h.
La velocidad de perfusión no debe exceder 3,0 ml/kg peso corporal/h (correspondiente a 0,09 g de aminoácidos, 0,21 g de glucosa y 0,09 g de lípidos/kg peso corporal/h).
La dosis diaria máxima recomendada es de 40 ml/kg peso corporal/día, que proporcionará 1,3 g de aminoácidos/kg peso corporal/día (correspondiente a 0,21 g de nitrógeno/kg peso corporal/día), 2,8 g de glucosa/kg peso corporal/día, 1,2 g de lípidos/kg peso corporal/día y una energía total de 27 kcal/kg peso corporal/día (correspondientes a 22 kcal/kg peso corporal/día de energía no proteica).
Población pediátrica
No se han realizado estudios con Finomel Peri en la población pediátrica.
Pacientes con insuficiencia renal/hepática
Usar con precaución en pacientes con insuficiencia hepática, incluyendo colestasis y/o enzimas hepáticas elevadas. Los parámetros de la función hepática deben ser controlados cuidadosamente.
Forma de administración
Uso intravenoso, perfusión en una vena periférica o central.
Para consultar las instrucciones de reconstitución del medicamento antes de la administración, ver sección E Precauciones especiales de eliminación y otras manipulaciones.
Si se utilizan venas periféricas para las perfusiones, debe tenerse en cuenta la osmolaridad de las soluciones, ya que puede producirse tromboflebitis. Debe evaluarse a diario el sitio de inserción del catéter para detectar signos localizados de tromboflebitis.
Para obtener información sobre la mezcla con otras perfusiones o sangre antes o durante la administración, ver sección C Incompatibilidades.
- INCOMPATIBILIDADES
Este medicamento no debe mezclarse con otros medicamentos para los que no se haya documentado la compatibilidad.
No debe mezclarse ni administrarse ceftriaxona junto con soluciones intravenosas que contengan calcio, incluido Finomel Peri.
Finomel Peri no debe administrarse junto con sangre a través de la misma vía de perfusión.
- SOBREDOSIS
En el caso de sobredosis, pueden producirse náuseas, vómitos, escalofríos, hiperglucemia y alteraciones de los electrolitos, así como signos de hipervolemia o acidosis. En estos casos, debe detenerse la perfusión inmediatamente.
Si se produce hiperglucemia, debe tratarse de acuerdo con la situación clínica, mediante la administración de insulina adecuada y/o el ajuste de la velocidad de perfusión. Asimismo, una sobredosis podría causar una sobrecarga de líquidos, desequilibrios de los electrolitos e hiperosmolaridad.
Si los síntomas persisten después de interrumpir la perfusión, puede considerarse la hemodiálisis, hemofiltración o hemodiafiltración.
- PRECAUCIONES ESPECIALES DE ELIMINACIÓN Y OTRAS MANIPULACIONES
Para abrir:
- Quite la sobrebolsa protectora.
- Deseche el sobrecito con el absorbente de oxígeno.
- Utilícela únicamente si la bolsa no está dañada, los sellos no permanentes están intactos (es decir, no se han mezclado los contenidos de los tres compartimentos), si la solución de aminoácidos y la solución de glucosa en sus respectivas cámaras están transparentes, incoloras o ligeramente amarillentas y libres de partículas visibles, y si la emulsión de lípidos es un líquido homogéneo de aspecto lechoso.
Para mezclar las cámaras:
- Asegúrese de que el producto está a temperatura ambiente cuando se rompan los sellos no permanentes.
- Enrolle manualmente la bolsa sobre sí misma, comenzando por la parte superior de la bolsa (extremo del colgador). (Ilustración 1)Los sellos no permanentes desaparecerán del lado cercano a las entradas. Siga enrollándola hasta que los sellos se abran aproximadamente hasta la mitad de su longitud. (Ilustración 2)
- Mezcle la bolsa invirtiéndola al menos 3 veces. (Ilustración 3)
- El aspecto tras la reconstitución es una emulsión homogénea de color lechoso.
Después de retirar la tapa protectora de la vía de medicación, se pueden añadir aditivos compatibles a través de la vía de medicación (ver la subsección “Adiciones”).
Retire la tapa protectora de la vía de perfusión y conecte el equipo de perfusión. Cuelgue la bolsa en un soporte de perfusión y lleve a cabo la perfusión mediante la técnica habitual. (Ilustración 4)
Tras abrir la bolsa, debe utilizarse el contenido inmediatamente. La bolsa abierta nunca debe guardarse para su posterior perfusión.
No vuelva a conectar una bolsa a medio utilizar. No conectar bolsas en serie para evitar que se produzca una embolia gaseosa.
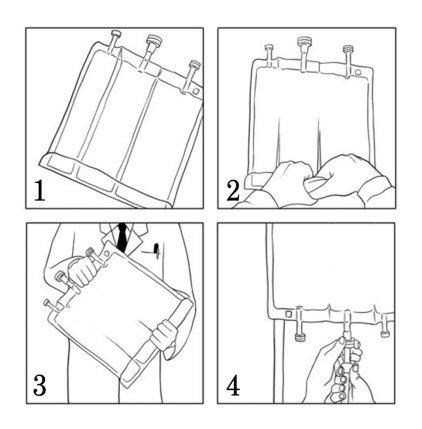
Adiciones
No deben realizarse adiciones a la bolsa sin comprobar antes la compatibilidad, ya que la formación de precipitados o la desestabilización de la emulsión de lípidos podrían producir oclusión vascular.
Las adiciones deben ser llevadas a cabo en condiciones asépticas.
Finomel Peri puede mezclarse con los siguientes aditivos:
- preparaciones multivitamínicas
- preparaciones de oligoelementos múltiples
- selenio
- zinc
- sal de sodio
- sal de potasio
- sal de magnesio
- sal de calcio
- sal de fosfato
La siguiente tabla indicativa de compatibilidades muestra posibles adiciones de productos con oligoelementos múltiples, como Nutryelt, y productos multivitamínicos, como Cernevit, así como genéricos de electrolitos y oligoelementos en cantidades definidas. La adición de electrolitos y oligoelementos clínicamente necesarios debe tener en cuenta las cantidades ya incluidas en la formulación inicial de la bolsa.
Aditivo | Contenido total después de la adiciónpara todos los tamaños de bolsa de Finomel Peri |
Nutryelt (composición por vial: zinc 153 µmol; cobre 4,7 µmol; manganeso 1,0 µmol; flúor 50 µmol; iodo 1,0 µmol; selenio 0,9 µmol; molibdeno 0,21 µmol; cromo 0,19 µmol; hierro 18 µmol) | 2 vialesa/bolsa |
Cernevit (composición por vial: vit. A (como retinol palmitato) 3500 UI, vit. D3 (colecalciferol) 220 UI, vit. E (alfa-tocoferol) 11,2 UI, vit. C (ácido ascórbico) 125 mg, vit. B1 (tiamina) 3,51 mg, vit. B2 (riboflavina) 4,14 mg, vit. B6 (piridoxina) 4,53 mg, vit. B12 (cianocobalamina) 6 µg, vit. B9 (ácido fólico) 414 µg, vit. B5 (ácido pantoténico) 17,25 mg, vit. B8 (biotina) 69 µg, vit. PP (nicotinamida) 46 mg) | 2 vialesb/bolsa |
Sodio | 138 mmol/l |
Potasio | 138 mmol/l |
Magnesio | 5 mmol/l |
Calcio | 4,6 mmol/l |
Fosfato (orgánico, como glicerofosfato sódico) o Fosfato (mineral, como fosfato potásico) | 18,5 mmol/l 9,2 mmol/l |
Selenio | 7,6 µmol/l |
Zinc | 0,31 mmol/l |
|
La compatibilidad puede variar entre productos de diferentes fuentes y se aconseja a los profesionales sanitarios llevar a cabo las comprobaciones adecuadas al mezclar Finomel Peri con otras soluciones parenterales.
Mezcle bien el contenido de la bolsa e inspeccione visualmente la mezcla. No debería haber signos de separación de fases de la emulsión. La mezcla es una emulsión homogénea de color blanco lechoso.
Al realizar las adiciones, debe medirse la osmolaridad final de la mezcla antes de administrarla a través de una vena periférica.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FINOMEL PERI EMULSION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 4,76 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 g / 3,5 g / 200 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 3,5 g / 200 g / 5,22 g / 1,88 g / 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 662 mg / 1,02 g / 4,76 g / 5,15 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 4,25 g / 300 g / 5,22 g / 1,54 g / 4,76 g / 1,53 g / 8,76 g / 4,08 g / 5,1 g / 6,2 g / 3,57 g / 3,4 g / 662 mg / 1,02 g / 5,78 g / 5,94 g / 6,16 g / 4,93 g / 17,6 g / 0,34 g / 9,78 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere receta
Médicos online para FINOMEL PERI EMULSION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FINOMEL PERI EMULSION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















