
BUDESONIDA NASAL ALDO-UNIÓN 100 microgramos/dosis SUSPENSION PARA PULVERIZACION NASAL


Cómo usar BUDESONIDA NASAL ALDO-UNIÓN 100 microgramos/dosis SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Budesonida Nasal Aldo-Unión y para qué se utiliza
- Qué necesita saber antes de empezar a usar Budesonida Nasal Aldo-Unión
- Cómo usar Budesonida Nasal Aldo-Unión
- Posibles efectos adversos
- Conservación de Budesonida Nasal Aldo-Unión
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Contenido del envase e información adicional.
Introducción
Prospecto: información para el paciente
Budesonida Nasal Aldo-Unión 100 microgramos/dosis
suspensión para pulverización nasal
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Budesonida Nasal Aldo-Union y para qué se utiliza
- Qué necesita saber antes de empezar a usar Budesonida Nasal Aldo-Unión
- Cómo usar Budesonida Nasal Aldo-Unión
- Posibles efectos adversos
- Conservación de Budesonida Nasal Aldo-Unión
- Contenido del envase e información adicional
1. Qué es Budesonida Nasal Aldo-Unión y para qué se utiliza
Este medicamento contiene como principio activo budesonida, que pertenece a un grupo de medicamentos llamados glucocorticoides y se emplea para reducir la inflamación de la mucosa nasal (parte interna de la nariz).
Budesonida Nasal Aldo-Unión se utiliza para el tratamiento de los síntomas de la rinitis alérgica estacional (“fiebre del heno”), así como de la rinitis perenne (todo el año) y la rinitis vasomotora.
2. Qué necesita saber antes de empezar a usar Budesonida Nasal Aldo-Unión
No use Budesonida Nasal Aldo-Unión
- Si es alérgico (hipersensible) a la budesonida o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Budesonida Nasal Aldo-Unión:
- Si padece o ha padecido tuberculosis.
- Si padece infecciones generalizadas o de las vías respiratorias no tratadas.
- Si presenta síntomas o signos de una infección localizada en las fosas nasales.
- Si previamente ha sido tratado con esteroides por vía sistémica.
Póngase en contacto con su médico si presenta visión borrosa u otras alteraciones visuales.
Budesonida Nasal Aldo-Unión le ha sido recetado para el tratamiento de su actual dolencia. No tome este medicamento para otras afecciones sin que se lo haya indicado su médico.
Debe evitarse el contacto del producto con los ojos. En caso de que se produzca contacto con los ojos, lávelos inmediatamente con agua abundante.
Niños y adolescentes
No se recomienda la administración de Budesonida Nasal Aldo-Unión en niños menores de 6 años.
Los niños de 6 o más años deben utilizar Budesonida Nasal Aldo-Unión solamente bajo la supervisión de un adulto con objeto de asegurar la correcta administración y que la dosis corresponde a la prescrita por el médico. El médico revisará periódicamente el crecimiento de los niños ya que este medicamento puede producir un retraso de su crecimiento.
Uso de Budesonida Nasal Aldo-Unión conotros medicamentos:
Informe a su médico si está tomando o ha tomado recientemente otros medicamentos, incluso los adquiridos sin receta.
Algunos medicamentos pueden aumentar los efectos de Budesonida Nasal Aldo-Unión, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No existe una evidencia clara de que este medicamento perjudique a la madre o al niño cuando se administra durante el embarazo o en periodo de lactancia.
Conducción y uso de máquinas
No hay indicios de que este medicamento pueda afectar a su capacidad de conducción.
Uso en deportistas
Si es deportista debe tener en cuenta que este medicamento contiene budesonida, que puede dar lugar a un resultado positivo en las pruebas de control de dopaje.
3. Cómo usar Budesonida Nasal Aldo-Unión
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis debe ser individualizada. Su médico le indicará la duración del tratamiento con Budesonida Nasal Aldo-Unión; no exceda la duración del tratamiento recomendada.
No debe compartir el pulverizador con otras personas debido al riesgo de contagio.
Rinitis alérgica estacional y perenne, rinitis no alérgica perenne:
Adultos: La dosis habitual de ataque es de dos aplicaciones en cada fosa nasal por la mañana. También puede administrarse en forma de una aplicación en cada fosa nasal, mañana y noche. Una vez conseguida una mejoría de los síntomas, su médico podrá reducirle la dosis.
Si usted padece rinitis alérgica estacional (“fiebre del heno”), deberá iniciar el tratamiento con Budesonida Nasal Aldo-Unión antes de que comience el periodo de alergia. Este medicamento no proporciona un alivio inmediato de los síntomas. Pueden ser necesarios varios días de tratamiento con Budesonida Nasal Aldo-Unión para que usted note un alivio de los mismos (en ocasiones hasta 2 semanas).
Este medicamento no alivia los síntomas oculares de la alergia. En caso de notar molestias en los ojos, su médico podrá recetarle algún otro medicamento para el alivio de dichos síntomas.
Si el alivio de los síntomas no se alcanza tras 3 semanas de tratamiento, deberá suspenderse la administración del preparado. Es conveniente administrar la dosis mínima efectiva.
En pacientes con rinitis alérgica perenne, una vez que se ha alcanzado un control adecuado de los síntomas, la dosis debe ser reducida gradualmente cada 2-4 semanas hasta que se siga manteniendo el efecto clínico esperado. Si los síntomas vuelven, la dosis puede ir incrementándose a la dosis de comienzo para luego pasar a la dosis en la que se había alcanzado un control adecuado de los síntomas.
Uso en niños y adolescentes
No se recomienda el uso de este medicamento en niños y adolescentes.
Instrucciones para la correcta administración del preparado:
Antes de la primera aplicación:
- Retirar el tapón de protección.
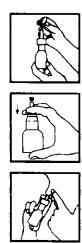 Agitar el conjunto frasco-aplicador.
Agitar el conjunto frasco-aplicador. - Accionar el pulsador las veces necesarias para que se llene al mecanismo de la bomba y pueda producirse una pulverización correcta.
Si no se utiliza diariamente, es necesario realizar una pulsación al aire antes de ser nuevamente empleado.
Modo de empleo. En cada aplicación:
- Sonarse con suavidad la nariz
- Retirar el tapón de protección
- Agitar el conjunto frasco-aplicador.
- Introducir el aplicador en una fosa nasal tapando la otra con el dedo.
- Inspirar y presionar enérgicamente sobre el fondo del frasco. Respirar por la boca y repetir la operación.
- Repetir el mismo proceso en la otra fosa nasal.
Después de su utilización, colocar el tapón de protección.
Limpieza:
Después de utilizar el pulverizador, para mantener limpia la boquilla se debe limpiar cuidadosamente el aplicador nasal con una toallita o un pañuelo limpio.
Si el pulverizador no funciona, es posible que la boquilla esté bloqueada. Nunca intente desbloquearlo o hacer más grande el orificio del pulsador con un alfiler u objeto punzante. Esto podría hacer que el pulverizador dejara de funcionar.
El aplicador nasal se quita tirando suavemente hacia arriba.
Si usa más Budesonida Nasal Aldo-Unióndel que debe:
Es importante que usted tome su dosis como le ha indicado su médico. Usted debe usar sólo lo que su médico le recomienda, el uso de más o menos dosis puede empeorar sus síntomas.
Consulte a su médico o a su farmacéutico, o llame al Servicio de Información Toxicológica, teléfono: 91 5620420, indicando el medicamento y la cantidad utilizada.
Si olvidó usarBudesonida Nasal Aldo-Unión:
No use una dosis doble para compensar las dosis olvidadas. Simplemente aplíquese la dosis siguiente como le ha sido prescrito.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han registrado las siguientes reacciones adversas, clasificadas por categorías de sistema orgánico y por orden de frecuencia decreciente:
Trastornos respiratorios, torácicos y mediastínicos:
- Frecuentes(≥1/100 a <1/10):
- Estornudos.
- Picor nasal.
- Sequedad nasal.
- Disfonía incluida ronquera
- Raras((≥1/10,000 a <1/1,000):
- Hemorragia nasal.
- Irritación de garganta.
- Muy raras(<1/10.000):
- Perforación del tabique nasal.
Trastornos de la piel y del tejido subcutáneo:
- Poco frecuentes(≥1/1000, <1/100):
Reacciones alérgicas cutáneas.
Trastornos oculares:
- Frecuencia no conocida (no puede estimarse la frecuencia a partir de los datos disponibles:
- Visión borrosa.
Si considera que alguno de estos efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Budesonida Nasal Aldo-Unión
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 30°C.
Conservar el envase en el embalaje exterior para protegerlo de la luz.
No congelar.
Vuelva a colocar la tapa protectora después de utilizar Budesonida Nasal Aldo-Unión.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de Cad. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de su farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional.
Composición deBudesonida Nasal Aldo-Unión
- El principio activo es budesonida. Cada aplicación contiene 100 microgramos de budesonida (2 mg/ml).
- Los demás componentes (excipientes) son: glucosa anhidra, celulosa microcristalina y carmelosa de sodio, polisorbato 80, edetato de sodio, sorbato de potasio (E-202), ácido clorhídrico y agua purificada.
Aspecto del producto y contenido del envase:
Budesonida Nasal Aldo-Unión es una suspensión blanca acuosa que se presenta en un envase de vidrio marrón que contiene 10 ml (200 dosis), provisto de una bomba dosificadora y de un adaptador nasal.
Titular de la autorización de comercialización y responsable de la fabricación:
Laboratorio ALDO-UNIÓN, S.L.
Baronesa de Maldà, 73
08950 Esplugues de Llobregat
BARCELONA – ESPAÑA
Fecha de la última revisión de este prospecto: Diciembre 2024
Otras fuentes de información
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/ “
- País de registro
- Precio medio en farmacia8.12 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BUDESONIDA NASAL ALDO-UNIÓN 100 microgramos/dosis SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 64 MCG/pulverizaciónPrincipio activo: BudesonidaFabricante: Laboratorio Aldo Union S.L.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 2 mg budesonida/ mlPrincipio activo: BudesonidaFabricante: M4 Pharma S.L.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1 mg budesonisa/mlPrincipio activo: BudesonidaFabricante: M4 Pharma S.L.Requiere receta
Médicos online para BUDESONIDA NASAL ALDO-UNIÓN 100 microgramos/dosis SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BUDESONIDA NASAL ALDO-UNIÓN 100 microgramos/dosis SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











