
BENEFIX 250 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar BENEFIX 250 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
BeneFIX 250UI polvo y disolvente para solución inyectable
BeneFIX 500UI polvo y disolvente para solución inyectable
BeneFIX 1000UI polvo y disolvente para solución inyectable
BeneFIX 1500UI polvo y disolvente para solución inyectable
BeneFIX 2000UI polvo y disolvente para solución inyectable
BeneFIX 3000UI polvo y disolvente para solución inyectable
nonacog alfa (factor IX de coagulación recombinante)
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es BeneFIX y para qué se utiliza
- Qué necesita saber antes de empezar a usar BeneFIX
- Cómo usar BeneFIX
- Posibles efectos adversos
- Conservación de BeneFIX
- Contenido del envase e información adicional
1. Qué es BeneFIX y para qué se utiliza
BeneFIX es un producto de factor IX de la coagulación inyectable obtenido por tecnología de ADN recombinante. La sustancia activa de BeneFIX es nonacog alfa. Las personas que nacen con hemofilia B (enfermedad de Christmas) no tienen suficiente factor IX para controlar las hemorragias. BeneFIX, actúa reponiendo el factor IX en los pacientes con hemofilia B para permitir que su sangre pueda coagularse.
BeneFIX se utiliza para el tratamiento y prevención de hemorragias en pacientes con hemofilia B (deficiencia de factor IX congénita) en todos los grupos de edad.
2. Qué necesita saber antes de empezar a usar BeneFIX
No use BeneFIX
- si es alérgico a nonacog alfa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si es alérgico a las proteínas de hámster.
Advertencias y precauciones
- Consulte a su médico o farmacéutico antes de empezar a usar BeneFIX.
- Consulte a su médico inmediatamente si su hemorragia no se detiene como cabría esperar.
- Se pueden producir reacciones alérgicas. El producto puede contener trazas de proteínas de hámster (ver No use BeneFIX). Con productos de factor IX, incluyendo BeneFIX se han producido reacciones anafilácticas (reacciones alérgicas graves) que potencialmente pueden poner en riesgo la vida. Los signos iniciales de las reacciones de alergia incluyen dificultad para respirar, respiración entrecortada, tumefacción o hinchazón, habón urticarial, picor, urticaria generalizada, opresión en el pecho, jadeo, presión arterial baja, visión borrosa y anafilaxis (reacción alérgica grave que puede causar dificultad al tragar y/o al respirar, enrojecimiento o hinchazón de la cara y/o de las manos).
- Si se produce una reacción de tipo alérgico o anafiláctico detenga inmediatamente la perfusión y contacte con un médico o busque inmediatamente ayuda médica de urgencia. En caso de reacciones alérgicas graves, debe considerarse la utilización de una terapia alternativa.
- La aparición de anticuerpos neutralizantes (inhibidores) es un acontecimiento poco frecuente en pacientes que han recibido tratamiento previo con productos que contienen factor IX. No obstante, como con todos los productos que contienen factor IX, debe ser vigilado cuidadosamente por si desarrolla inhibidores del factor IX mientras está en tratamiento con BeneFIX.
- Estudios han mostrado una relación entre la aparición de un inhibidor del factor IX y reacciones alérgicas. Por ello, si experimenta reacciones alérgicas como las descritas arriba, deberá ser examinado para determinar la presencia de un inhibidor. Se debe tener en cuenta que los pacientes con un inhibidor del factor IX pueden presentar un riesgo aumentado de anafilaxis en un futuro tratamiento con BeneFIX.
- La producción de factor IX en el organismo está controlada por el gen del factor IX. Los pacientes con mutaciones específicas del gen del factor IX como una delección importante, podrán tener más probabilidades de desarrollar un inhibidor del factor IX y/o padecer reacciones alérgicas. Por lo tanto, si se sabe que tiene dicha mutación, su médico puede monitorizarle más de cerca por si hubiera signos de una reacción alérgica, especialmente cuando empiece a tomar BeneFIX por primera vez.
- Dado el riesgo de reacciones alérgicas con factor IX, las administraciones iniciales de BeneFIX deben realizarse bajo supervisión médica, donde pueda proporcionarse un cuidado médico adecuado para las reacciones alérgicas.
- Aún en ausencia de inhibidor del factor IX, pueden ser necesarias dosis mayores de BeneFIX que las que se precisan para otros de factor IX derivados de plasma que haya recibido anteriormente. Por consiguiente, se tiene que realizar una estrecha monitorización de la actividad de factor IX en plasma (que mide la capacidad de su sangre para formar coágulos) con objeto de ajustar la dosis adecuadamente. Si la hemorragia no se controla con la dosis recomendada contacte con su médico.
- Si sufre de enfermedad del hígado o del corazón o si ha sufrido recientemente una intervención quirúrgica, existe un mayor riesgo de complicaciones de coagulación de la sangre.
- Se ha comunicado una alteración de los riñones (síndrome nefrótico) tras la administración de dosis altas de factor IX derivado del plasma en pacientes con hemofilia B con inhibidores del factor IX y antecedentes de reacciones alérgicas.
- No se han obtenido datos suficientes de los ensayos clínicos de pacientes no tratados previamente (pacientes que nunca han recibido una perfusión con factor IX) con BeneFIX.
- Se recomienda que cada vez que use BeneFIX registre el nombre y el número de lote del producto. Puede usar una de las etiquetas despegables que se encuentran en el vial para dejar constancia del número de lote en su diario o para notificar cualquier reacción adversa.
Otros medicamentos y BeneFIX
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, sólo debe recibir BeneFIX por indicación específica de su médico. Se desconoce si BeneFIX puede causar daño al feto cuando se administra a mujeres embarazadas. Si está en periodo de lactancia o se queda embarazada, el médico puede aconsejarle que suspenda el tratamiento con BeneFIX.
Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de BeneFIX sobre la capacidad para conducir y utilizar máquinas es nula.
BeneFIX contiene sodio
Tras la reconstitución, BeneFIX contiene 0,2 mmol de sodio (4,6 mg) por vial; esto es, esencialmente “exento de sodio”. Sin embargo, según su peso corporal y su dosis de BeneFIX, podría recibir múltiples viales. Esto se debe tener en cuenta si usted sigue una dieta baja en sal.
3. Cómo usar BeneFIX
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico decidirá la dosis de BeneFIX que recibirá. Esta dosis y su duración dependerán de sus necesidades individuales de tratamiento sustitutivo con factor IX y de la rapidez con que su organismo utilice el factor IX lo que será comprobado regularmente. Podrá apreciar una diferencia en la dosis que recibe si cambia de un producto de factor IX derivado de plasma a BeneFIX.
Su médico puede modificar la dosis de BeneFIX que reciba a lo largo del tratamiento.
Reconstitución y administración
Las instrucciones facilitadas a continuación son una guía para la reconstitución y administración de BeneFIX. Los pacientes deben seguir las instrucciones de venopunción específicas indicadas por su médico.
BeneFIX se administra mediante perfusión intravenosa (IV) después de la reconstitución del polvo para inyección con el disolvente incluido en la jeringa precargada (solución de cloruro de sodio).
Lávese siempre las manos antes de realizar los procedimientos siguientes. Durante el procedimiento de reconstitución debe seguirse una técnica aséptica (que significa limpio y libre de gérmenes).
Reconstitución:
BeneFIX se administrará por perfusión intravenosa (IV) después de la reconstitución con disolvente estéril para inyección.
- Permita que el vial liofilizado de BeneFIX y la jeringa precargada de disolvente alcancen la temperatura ambiente.
- Retire la cápsula de cierre del vial de BeneFIX para que quede visible la parte central del tapón de goma.
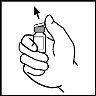
- Limpie la parte superior del vial con la torunda empapada en alcohol que se proporciona o utilice otra solución antiséptica y espere a que se seque. Después de limpiar, no toque el tapón de goma con su mano y evite que toque ninguna superficie.
- Retire la tapa protectora del envase de plástico transparente del adaptador. No saque el adaptador del envase.
- Coloque el vial sobre una superficie plana. Mientras sujeta el adaptador en su envase, colóquelo sobre el vial. Presiónelo firmemente hasta que el adaptador encaje en lo alto del vial, con la punta del adaptador penetrando en el tapón del vial.
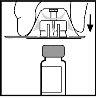
- Retire el envase del adaptador y deséchelo.
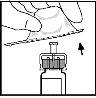
- Encaje la barra del émbolo a la jeringa del disolvente empujándola y girándola firmemente.
- Rompa la tapa de plástico resistente a la manipulación partiendo la perforación del capuchón. Esto se realiza doblando el capuchón hacia arriba y abajo hasta que se rompa la perforación. No toque el interior del capuchón ni la punta de la jeringa. El capuchón puede necesitar ser reemplazado (si BeneFIX reconstituido no es administrado inmediatamente), por tanto deséchelo colocándolo en su parte superior.
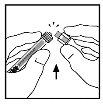
- Coloque el vial en una superficie plana. Conecte la jeringa del disolvente al adaptador del vial insertando la punta de la jeringa en la apertura del adaptador mientras empuja y gira firmemente la jeringa en el sentido de las agujas del reloj hasta que encaje perfectamente.
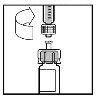
- Haga descender lentamente el émbolo para inyectar todo el disolvente en el vial de BeneFIX.
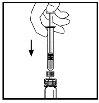
- Con la jeringa aún conectada al adaptador, gire suavemente el vial hasta que se disuelva el polvo.
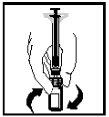
- La solución final debe ser examinada visualmente en busca de partículas finas antes de la administración. La solución debe aparecer transparente e incolora.
Nota: Si utiliza más de un vial de BeneFIX para perfusión, cada vial debe ser reconstituido de acuerdo con las instrucciones anteriores. La jeringa de disolvente deberá desecharse, dejando el adaptador del vial en su sitio y a continuación se utilizará una jeringa grande tipo Luer Lock (un dispositivo que conecta la jeringa al vial) para retirar el contenido reconstituido de cada vial individual.
- Gire el vial asegurándose de que el émbolo de la jeringa ha descendido por completo. Aspire lentamente toda la solución al interior de la jeringa.
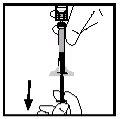
- Libere la jeringa del adaptador empujando y girando lentamente la jeringa en el sentido de las agujas del reloj. Deseche el vial unido al adaptador.
Nota: Si la solución no va a ser utilizada inmediatamente, el capuchón debe ser reemplazado cuidadosamente. No toque la punta de la jeringa ni el interior del capuchón.
BeneFIX, debe administrarse inmediatamente después de su reconstitución, o bien dentro de las 3 horas siguientes. La solución reconstituida puede almacenarse a temperatura ambiente antes de la administración.
Administración (Inyección intravenosa):
BeneFIX debe administrarse mediante la jeringa precargada de disolvente que se suministra o mediante una jeringa de plástico desechable estéril tipo Luer Lock. Además, la solución debe extraerse del vial utilizando el adaptador del vial.
BeneFIX debe ser inyectado intravenosamente durante varios minutos. El médico le podrá cambiar la velocidad de la perfusión recomendada a fin de que le resulte más cómoda.
Se han notificado casos de aglutinación de hematíes en el tubo o en la jeringa durante la administración de BeneFIX. No se han comunicado reacciones adversas en relación con esta observación. Para reducir al mínimo la posibilidad de aglutinación, es importante limitar la cantidad de sangre que entra en el tubo. La sangre no debe entrar en la jeringa. Si se observa aglutinación de hematíes en el tubo o en la jeringa, deseche todo este material (tubo, jeringa y solución de BeneFIX) y reanude la administración con un nuevo envase.
Dado que el uso de BeneFIX en perfusión continua (gota a gota) no ha sido evaluado, BeneFIX no debe mezclarse con soluciones para perfusión o administrarse gota a gota.
Deseche la solución no utilizada, los viales vacíos y las agujas y jeringas utilizadas en un recipiente adecuado para eliminar desechos que puedan provocar lesiones, si no se manejan adecuadamente.
Si usa más BeneFIX del que debe
Si se inyecta una cantidad de BeneFIX, superior a la recomendada por el médico, póngase en contacto con él inmediatamente.
Si interrumpe el tratamiento con BeneFIX
No interrumpa el tratamiento con BeneFIX sin consultar con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones de hipersensibilidad/alergia
Es posible que se produzcan reacciones de hipersensibilidad de tipo alérgico con BeneFIX. Tales reacciones pueden consistir en hinchazón de la cara o la garganta, escozor y picor en el lugar de perfusión, escalofríos, rubefacción (enrojecimiento de la piel), prurito, dolor de cabeza, habón urticarial, disminución de la presión arterial, letargo, náuseas, inquietud, aumento de la frecuencia cardiaca, opresión en el pecho, hormigueo, vómitos, sibilancias (ruidos al respirar). En algunos casos, estas reacciones han progresado hasta convertirse en anafilaxias graves. Pueden aparecer reacciones alérgicas junto con el desarrollo de inhibidores del factor IX (ver también “Advertencias y precauciones”).
Estas reacciones pueden potencialmente poner en riesgo la vida. Si se producen reacciones anafilácticas/alérgicas, detenga inmediatamente la perfusión y contacte con un médico o busque inmediatamente ayuda médica de urgencia. El tratamiento requerido dependerá de la naturaleza y gravedad de los efectos adversos (ver también “Advertencias y precauciones”).
Desarrollo de inhibidores
Los pacientes con hemofilia B pueden desarrollar anticuerpos neutralizantes (inhibidores) al factor IX. Si esto ocurriera, un signo puede ser un incremento en la cantidad de BeneFIX que normalmente se requiere para tratar una hemorragia, y/o que la hemorragia continúe después del tratamiento. En estos casos, se recomienda contactar con un centro especializado en hemofilia. Su médico puede monitorizarle para controlar el desarrollo de inhibidores (ver “Advertencias y precauciones”).
Se ha notificado una alteración de los riñones tras la administración de dosis altas de factor IX derivado del plasma para la inducción de inmunotolerancia en pacientes con hemofilia B con inhibidores de factor IX y antecedentes de reacciones alérgicas (ver también “Advertencias y precauciones”).
Acontecimientos trombóticos
BeneFIX puede aumentar el riesgo de trombosis (coágulos de sangre anormales) en su organismo, si tiene factores de riesgo para el desarrollo de coágulos de sangre, incluyendo un catéter venoso permanente. Se han notificado casos graves de coágulos de sangre, incluyendo coágulos de sangre con riesgo para la vida en bebés críticamente enfermos, mientras recibían BeneFIX en perfusión continua a través de un catéter venoso central. También se han notificado casos de tromboflebitis periférica (dolor y enrojecimiento de las venas) y trombosis venosa profunda (coágulos de sangre en las extremidades); en la mayoría de estos casos, BeneFIX se había administrado por medio de perfusión continua, que es un método de administración no aprobado.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza
- Tos
- Fiebre
Efectos adversos frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- Reacciones de hipersensibilidad o alergia
- Mareos, alteración del sentido del gusto
- Flebitis (dolor y enrojecimiento de las venas), rubefacción (enrojecimiento de la piel)
- Vómitos, náuseas
- Erupción cutánea, habón urticarial
- Molestia en el pecho (incluido dolor en el pecho)
- Reacción en el lugar de perfusión (incluidos picor y enrojecimiento en el lugar de perfusión), dolor y molestia en el lugar de perfusión
Efectos adversos poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- Aparición de anticuerpos neutralizantes (inhibidores)
- Celulitis en el lugar de perfusión (dolor y enrojecimiento de la piel)
- Somnolencia, temblores
- Alteración de la visión (incluidas visión borrosa, aparición de manchas o luces)
- Aumento de la frecuencia cardiaca, disminución de la presión arterial
- Infarto renal (interrupción del suministro sanguíneo al riñón)
Efectos adversos de frecuencia no conocida(no puede estimarse a partir de los datos disponibles)
- Reacción anafiláctica
- Acontecimientos trombóticos (coágulos de sangre anormales)
- Ausencia de respuesta al tratamiento (los episodios de sangrado no se pueden detener ni evitar)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de BeneFIX
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el estuche y en la etiqueta del vial. La fecha de caducidad es el último día del mes que se indica.
BeneFIX, debe conservarse por debajo de 30ºC y debe ser usado antes de la fecha de caducidad que aparece en la etiqueta.
No congelar el producto para evitar que se dañe la jeringa precargada.
El producto reconstituido debe utilizarse inmediatamente o en un plazo de 3 horas.
No utilice este medicamento si observa que la solución no es transparente o incolora.
Para la reconstitución sólo debe utilizarse la jeringa precargada que se incluye en el estuche. Para la administración, pueden emplearse otras jeringas desechables estériles.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de BeneFIX
- El principio activo es nonacog alfa (factor IX de coagulación recombinante). Cada vial de BeneFIX contiene nominalmente 250, 500, 1000, 1500, 2000 ó 3000 UI de nonacog alfa.
- Los demás componentes son sacarosa, glicina, L-histidina, polisorbato 80. Se incluye un disolvente para la reconstitución (solución de cloruro de sodio al 0,234%).
- Después de la reconstitución con el disolvente incluido, (solución de cloruro de sodio al 0,234%) cada vial contiene 50, 100, 200, 300, 400 ó 600 UI respectivamente (ver tabla 1) de nonacog alfa por 1 ml de la solución para inyección preparada.
Tabla 1. Concentración de BeneFIX por ml de solución preparada
Cantidad de BeneFIX por Vial | Cantidad de BeneFIX por 1ml de solución preparada para inyección |
250 UI | 50 UI |
500 UI | 100 UI |
1000 UI | 200 UI |
1500 UI | 300 UI |
2000 UI | 400 UI |
3000 UI | 600 UI |
Aspecto del producto y contenido del envase
BeneFIX se suministra como un polvo para inyección en un vial de vidrio y un disolvente suministrado en una jeringa precargada.
El pack contiene:
- un vial con BeneFIX 250, 500, 1000, 1500, 2000 o 3000 UI polvo
- una jeringa precargada con disolvente, 5 ml de solución de cloruro de sodio al 0,234% para la reconstitución
- un adaptador estéril para la reconstitución
- un sistema de perfusión estéril
- dos torundas con alcohol
- un apósito adhesivo
- una compresa de gasa
Titular de la autorización de comercialización
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Bélgica
Responsable de la fabricación
Wyeth Farma S.A
Autovía del Norte.A-1, Km. 23. Desvío Algete, Km. 1, 28700 San Sebastián de los Reyes, Madrid
España
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel. +3705 2514000 |
???????? ??????? ?????????? ????, ???? ???????? ???.: +359 2 970 4333 | Magyarország Pfizer Kft. Tel.: + 36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf.: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλ?δα Pfizer Ελλ?ς Α.Ε. Τηλ: +30 210 6785800 | Polska Pfizer Polska Sp. z o.o., Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: + 386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf. Sími: + 354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κ?προς Pfizer Ελλ?ς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 | |
Fecha de la última revisión de este prospecto: 03/2025.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BENEFIX 250 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: Factor de coagulación de la sangre humana IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requiere recetaForma farmacéutica: INYECTABLE, 2.000 UIPrincipio activo: Factor de coagulación de la sangre humana IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requiere recetaForma farmacéutica: INYECTABLE, 250 UIPrincipio activo: Factor de coagulación de la sangre humana IXFabricante: Swedish Orphan Biovitrum Ab (Publ)Requiere receta
Médicos online para BENEFIX 250 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BENEFIX 250 UI, POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















