
БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Запитайте лікаря про рецепт на БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Інструкція із застосування БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Введення
Опис: інформація для користувача
BeneFIX 250МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
BeneFIX 500МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
BeneFIX 1000МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
BeneFIX 1500МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
BeneFIX 2000МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
BeneFIX 3000МО одnorазове застосування порошок і розчинник для ін'єкційного розчину
нонаког альфа (рекомбінантний фактор згортання крові IX)
Прочитайте уважно весь опис перед тим, як почати використовувати цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
- Цей лікарський засіб призначений лише вам, і не слід давати його іншим людям, навіть якщо вони мають相同ні симптоми, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке BeneFIX і для чого він використовується
- Що вам потрібно знати перед тим, як почати використовувати BeneFIX
- Як використовувати BeneFIX
- Можливі побічні ефекти
- Зберігання BeneFIX
- Зміст упаковки та додаткова інформація
1. Що таке BeneFIX і для чого він використовується
BeneFIX - це продукт фактору згортання крові IX для ін'єкцій, отриманий за допомогою технології рекомбінантної ДНК. Активна речовина BeneFIX - нонаког альфа. Люди, які народилися з гемофілією Б (хворобою Крісмаса), не мають достатньої кількості фактору IX для контролю кровотеч. BeneFIX діє шляхом заміни фактору IX у пацієнтів з гемофілією Б, щоб їхня кров могла згортатися.
BeneFIX використовується для лікування та профілактики кровотеч у пацієнтів з гемофілією Б (вродженою недостатністю фактору IX) у всіх вікових групах.
2. Що вам потрібно знати перед тим, як почати використовувати BeneFIX
Не використовуйте BeneFIX
- якщо ви алергічні на нонаког альфа або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6);
- якщо ви алергічні на білки хом'яків.
Попередження та обережність
- Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як почати використовувати BeneFIX.
- Проконсультуйтеся з вашим лікарем негайно, якщо ваша кровотеча не зупиняється так, як очікувалося.
- Можуть виникнути алергічні реакції. Продукт може містити сліди білків хом'яків (див. Не використовуйте BeneFIX). З продуктами фактору IX, включаючи BeneFIX, були зареєстровані анафілактичні реакції (тяжкі алергічні реакції), які потенційно можуть загрожувати життю. Перші ознаки алергічних реакцій включають труднощі з диханням, переривчасте дихання, набухання або запалення, кропив'янка, свербіж, загальна кропив'янка, тиск на грудній клітці, задишка, низький тиск, розмитий зір та анафілаксія (тяжка алергічна реакція, яка може викликати труднощі з ковтанням і/або диханням, червоність або набухання обличчя та/або рук).
- Якщо виникає алергічна реакція або анафілактична реакція негайно зупиніть інфузію та зверніться до лікаря або шукайте негайну медичну допомогу. У разі тяжких алергічних реакцій слід розглянути можливість використання альтернативної терапії.
- Виникнення нейтралізуючих антитіл (інгібіторів) - це рідкісне явище у пацієнтів, які раніше отримували лікування продуктами, що містять фактор IX. Однак, як і з усіма продуктами, що містять фактор IX, слід уважно спостерігати за тим, чи розвиваються інгібітори фактору IX під час лікування BeneFIX.
- Дослідження показали зв'язок між виникненням інгібітора фактору IX та алергічними реакціями. Тому, якщо ви відчуваєте алергічні реакції, як описано вище, слід провести обстеження на наявність інгібітора. Слід враховувати, що пацієнти з інгібітором фактору IX можуть мати підвищений ризик анафілаксії при майбутньому лікуванні BeneFIX.
- Виробництво фактору IX в організмі контролюється геном фактору IX. Пацієнти з певними мутаціями гену фактору IX, такими як велике видалення, можуть мати більшу ймовірність розвитку інгібітора фактору IX та/або алергічних реакцій. Тому, якщо вам відомо про таку мутацію, ваш лікар може спостерігати за вами більш уважно на предмет ознак алергічної реакції, особливо коли ви починаєте приймати BeneFIX вперше.
- У зв'язку з ризиком алергічних реакцій з фактором IX, перші інфузії BeneFIX повинні проводитися під медичним наглядом, де можна забезпечити належну медичну допомогу у разі алергічних реакцій.
- Навіть у відсутності інгібітора фактору IX можуть знадобитися більші дози BeneFIX, ніж ті, які необхідні для інших продуктів фактору IX, отриманих з плазми, які ви раніше приймали. Тому слід проводити тісний моніторинг активності фактору IX в плазмі (що вимірює здатність вашої крові утворювати згустки) з метою корекції дози. Якщо кровотеча не зупиняється при рекомендованій дозі, зверніться до вашого лікаря.
- Якщо ви страждаєте на захворювання печінки або серця чи якщо ви недавно перенесли хірургічну операцію, існує більший ризик ускладнень згортання крові.
- Було повідомлено про порушення нирок (нефротичний синдром) після введення високих доз фактору IX, отриманого з плазми, у пацієнтів з гемофілією Б з інгібіторами фактору IX та алергічними реакціями.
- Не було отримано достатніх даних з клінічних досліджень у пацієнтів, які не були раніше лікувані (пацієнти, які ніколи не отримували інфузії фактору IX) з BeneFIX.
- Рекомендується, щоб кожен раз, коли ви використовуєте BeneFIX, реєстрували назву та номер партії продукту. Ви можете використовувати одну з відокремлених етикеток, які знаходяться на флаконі, щоб зробити запис про номер партії у вашому журналі або для повідомлення про будь-яку побічну реакцію.
Інші лікарські засоби та BeneFIX
Повідомте вашого лікаря або фармацевта, якщо ви використовуєте, нещодавно використовували або можете використовувати будь-який інший лікарський засіб.
Вагітність, лактація та фертильність
Якщо ви вагітні або перебуваєте в період лактації, вважаєте, що можете бути вагітні або плануєте вагітність, ви повинні приймати BeneFIX лише за спеціальною вказівкою вашого лікаря. Не відомо, чи може BeneFIX викликати шкоду плоду при введенні вагітним жінкам. Якщо ви перебуваєте в період лактації або вагітності, лікар може порадити вам припинити лікування BeneFIX.
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як використовувати цей лікарський засіб.
Водіння транспортних засобів та використання машин
Вплив BeneFIX на здатність водіння транспортних засобів та використання машин є нульовим.
BeneFIX містить натрій
Після реконституції BeneFIX містить 0,2 ммоль натрію (4,6 мг) на флакон; тобто, він практично "не містить натрію". Однак, залежно від вашої ваги та дози BeneFIX, ви можете отримувати кілька флаконів. Це слід враховувати, якщо ви дотримуєтеся дієти з низьким вмістом солі.
3. Як використовувати BeneFIX
Слідуйте точно інструкціям з введення цього лікарського засобу, вказаним вашим лікарем або фармацевтом. У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом.
Ваш лікар визначить дозу BeneFIX, яку ви отримуватимете. Ця доза та тривалість лікування залежатимуть від ваших індивідуальних потреб лікування фактором IX та швидкості, з якою ваш організм використовує фактор IX, що буде регулярно перевірятися. Ви можете помітити різницю в дозі, яку ви отримуватимете, якщо зміните продукт фактору IX, отриманий з плазми, на BeneFIX.
Ваш лікар може змінити дозу BeneFIX, яку ви отримуватимете, протягом лікування.
Реконституція та введення
Інструкції, надані нижче, є керівництвом для реконституції та введення BeneFIX. Пацієнти повинні слідувати інструкціям з венепункції, вказаним їхнім лікарем.
BeneFIX вводиться шляхом внутрішньовенної інфузії (ВВ) після реконституції порошку для ін'єкцій з розчинником, включеним до попередньо заповненої шприцу (розчин хлориду натрію).
Ви завжди повинні мити руки перед тим, як виконувати наступні процедури. Під час процедури реконституції слід дотримуватися асептичної техніки (що означає чистоту та відсутність мікробів).
Реконституція:
BeneFIX буде введено шляхом внутрішньовенної інфузії (ВВ) після реконституції з стерильним розчинником для ін'єкцій.
- Дозвольте флакону з лioфілізованим BeneFIX та попередньо заповненому шприцу з розчинником досягти кімнатної температури.
- Видаліть кришку з флакону BeneFIX, щоб стала видна центральна частина гумової пробки.
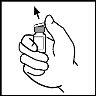
- Очистіть верхню частину флакону ватним тампоном, змоченим в алкоголі, який надано, або використовуйте інший антисептичний розчин та чекайте, поки він висохне. Після очищення не торкайтеся гумової пробки вашою рукою та уникайте її контакту з будь-якою поверхнею.
- Видаліть захисну кришку з пластикового прозорого контейнера адаптера. Не виймайте адаптер з контейнера.
- Помістіть флакон на плоску поверхню. Тримаючи адаптер у контейнері, помістіть його на флакон. Натисніть його сильно, поки адаптер не закріпиться на верху флакону, з кінчиком адаптера, який проникає в гумовую пробку флакону.
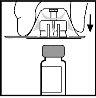
- Видаліть контейнер адаптера та викиньте його.
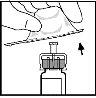
- Закріпіть поршень шприцу, надавши та повернувши його сильно.
- Зламайте пластикову кришку, розламавши перфорування кришки. Це здійснюється шляхом згинання кришки вгору та вниз, поки не зламається перфорування. Не торкайтеся внутрішньої частини кришки чи кінчика шприцу. Кришка може потребувати заміни (якщо реконструйований BeneFIX не буде введено негайно), тому викиньте її, помістивши її на верх.
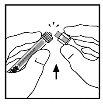
- Помістіть флакон на плоску поверхню. Підключіть шприц з розчинником до адаптера флакону, вставивши кінчик шприцу в отвір адаптера, поки він не закріпиться сильно, повертаючи шприц за годинниковою стрілкою.
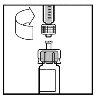
- Повільно опустіть поршень, щоб ввести весь розчинник у флакон з BeneFIX.
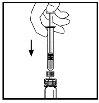
- З шприцем, все ще підключеним до адаптера, повільно поверніть флакон, поки порошок не розчиниться.
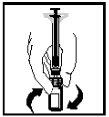
- Останній розчин повинен бути візуально перевірений на наявність дрібних частинок перед введенням. Розчин повинен виглядати прозорим та безбарвним.
Примітка: Якщо ви використовуєте більше одного флакону BeneFIX для інфузії, кожен флакон повинен бути реконструйований згідно з інструкціями вище. Шприц з розчинником слід викинути, залишаючи адаптер флакону на місці, а потім використовувати великий шприц типу Luer Lock (пристрій, який підключає шприц до флакону) для видалення реконструйованого вмісту кожного окремого флакону.
- Поверніть флакон, переконавшись, що поршень шприцу опустився повністю. Повільно аспіруйте весь розчин до шприцу.
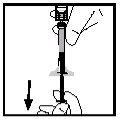
- Відпустіть шприц від адаптера, повільно повертаючи шприц проти годинникової стрілки. Викиньте флакон, підключений до адаптера.
Примітка: Якщо розчин не буде використовуватися негайно, кришка повинна бути відновлена обережно. Не торкайтеся кінчика шприцу чи внутрішньої частини кришки.
BeneFIX повинен бути введено негайно після реконституції або протягом 3 годин після цього. Реконструйований розчин може зберігатися при кімнатній температурі перед введенням.
Введення (внутрішньовенна ін'єкція):
BeneFIX повинен бути введено шляхом внутрішньовенної інфузії (ВВ) за допомогою попередньо заповненого шприцу з розчинником або за допомогою стерильного пластикового шприцу типу Luer Lock. Крім того, розчин повинен бути видалений з флакону за допомогою адаптера флакону.
BeneFIX повинен бути введено внутрішньовенно протягом кількох хвилин. Лікар може змінити швидкість інфузії, щоб зробити її більш комфортною для вас.
Було повідомлено про випадки аглютинації еритроцитів у трубці або шприці під час введення BeneFIX. Не було повідомлено про побічні реакції, пов'язані з цією спостереженням. Для мінімізації можливості аглютинації важливо обмежити кількість крові, яка потрапляє до трубки. Кров не повинна потрапляти до шприцу. Якщо ви спостерігаєте аглютинацію еритроцитів у трубці або шприці, викиньте весь матеріал (трубку, шприц та розчин BeneFIX) та відновіть введення з нового набору.
Оскільки використання BeneFIX у вигляді безперервної інфузії (крапля за краплею) не було оцінено, BeneFIX не повинен змішуватися з розчинами для інфузії або вводитися крапля за краплею.
Викиньте невикористаний розчин, порожні флакони та голки та шприци, які були використані, у відповідний контейнер для видалення відходів, які можуть викликати травму, якщо вони не обробляються належним чином.
Якщо ви використовуєте більше BeneFIX, ніж потрібно
Якщо ви ввели більшу кількість BeneFIX, ніж рекомендовано лікарем, негайно зверніться до нього.
Якщо ви припиняєте лікування BeneFIX
Не припиняйте лікування BeneFIX без консультації з вашим лікарем.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, зверніться до вашого лікаря, фармацевта або медсестри.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Алергічні реакції/гіпersenситивність
Можливі алергічні реакції на BeneFIX. Такі реакції можуть включати набряк обличчя або горла, свербіж і печію в місці інфузії, озноб, гіперемію (червоність шкіри), свербіж, головний біль, кропив'янку, зниження артеріального тиску, сонливість, нудоту, неспокій, підвищення частоти серцевих скорочень, відчуття тиску в грудній клітці, оніміння, блювоту, свистячий дихання. В деяких випадках ці реакції прогресували до важких анафілактичних реакцій. Алергічні реакції можуть виникати разом з розвитком інгібіторів фактора IX (див. також «Попередження та обережність»).
Ці реакції можуть потенційно загрожувати життю. Якщо виникають анафілактичні/алергічні реакції, негайно припиніть інфузію та зверніться до лікаря або шукайте негайної медичної допомоги. Необхідне лікування залежатиме від природи та тяжкості побічних ефектів (див. також «Попередження та обережність»).
Розвиток інгібіторів
Пацієнти з гемофілією Б можуть розвивати нейтралізуючі антитіла (інгібітори) до фактора IX. Якщо це відбувається, ознакою може бути збільшення кількості BeneFIX, необхідної для лікування кровотечі, та/або тривалість кровотечі після лікування. У таких випадках рекомендується звернутися до спеціалізованого центру з лікування гемофілії. Ваш лікар може контролювати розвиток інгібіторів (див. «Попередження та обережність»).
Було повідомлено про порушення функції нирок після введення високих доз фактора IX, отриманого з плазми, для індукції імунотолерантності у пацієнтів з гемофілією Б з інгібіторами фактора IX та алергічними реакціями (див. також «Попередження та обережність»).
Тромботичні події
BeneFIX може збільшувати ризик тромбозу (анормальних кров'яних згортань) в організмі, якщо у вас є фактори ризику розвитку тромбозу, включаючи постійний венозний катетер. Було повідомлено про важкі випадки тромбозу, включаючи тромбоз з ризиком для життя у критично хворих новонароджених, які отримували BeneFIX під час безперервної інфузії через центральний венозний катетер. Також були повідомлені випадки периферичної тромбофлебіту (болю та гіперемії вен) та глибокої венозної тромбози (тромбозу в кінцівках); у більшості цих випадків BeneFIX вводили шляхом безперервної інфузії, яка є незатвердженим методом введення.
Дуже часті побічні ефекти(можуть виникати у понад 1 з 10 осіб)
- Головний біль
- Кашель
- Гарячка
Часті побічні ефекти(можуть виникати у до 1 з 10 осіб)
- Алергічні реакції або гіпersenситивність
- Головокружіння, порушення смаку
- Флебіт (болю та гіперемія вен), гіперемія (червоність шкіри)
- Блювота, нудота
- Висипання на шкірі, кропив'янка
- Болю в грудній клітці (включаючи біль у грудній клітці)
- Реакція в місці інфузії (включаючи свербіж та гіперемію в місці інфузії), біль та дискомфорт в місці інфузії
Побічні ефекти незнайомої частоти(не можуть бути оцінені з наявних даних)
- Анафілактична реакція
- Тромботичні події (анормальні кров'яні згортання)
- Відсутність реакції на лікування (епізоди кровотечі не можуть бути зупинені чи попереджені)
Звіт про побічні ефекти
Якщо ви відчуваєте будь-які побічні ефекти, зверніться до свого лікаря, фармацевта або медсестри, навіть якщо це можливі побічні ефекти, які не перелічені в цьому листку інструкцій. Ви також можете повідомити про них безпосередньо через національну систему повідомлення, вказану в додатку V. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти забезпечити більш повну інформацію про безпеку цього лікарського засобу.
5. Зберігання BeneFIX
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на упаковці та на етикетці флакона. Термін придатності — останній день місяця, вказаного на упаковці.
BeneFIX повинен зберігатися при температурі нижче 30°C та використовуватися до закінчення терміну придатності, вказаного на етикетці.
Не заморожуйте продукт, щоб уникнути пошкодження попередньо наповненої серингі.
Перевстановлений продукт повинен бути використаний негайно або протягом 3 годин.
Не використовуйте цей лікарський засіб, якщо ви помітили, що розчин не є прозорим або безбарвним.
Для відновлення слід використовувати лише попередньо наповнену серингу, яка входить до складу упаковки. Для введення можна використовувати інші стерильні серингі.
Лікарські засоби не повинні викидатися у водопровідні труби чи сміття. Спитайте у свого фармацевта, як позбутися упаковки та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад BeneFIX
- Активний інгредієнт — нонаког альфа (рекомбінантний коагуляційний фактор IX). Кожна флакон BeneFIX містить номінально 250, 500, 1000, 1500, 2000 або 3000 ОД фактора IX.
- Інші компоненти — сукроза, гліцин, L-гістидин, полісорбат 80. У складі також є розчинник для відновлення (розчин хлориду натрію 0,234%).
- Після відновлення з розчинником, який входить до складу упаковки (розчин хлориду натрію 0,234%), кожна флакон містить 50, 100, 200, 300, 400 або 600 ОД нонакога альфа на 1 мл розчину для ін'єкції.
Таблиця 1. Концентрація BeneFIX на мл відновленого розчину для ін'єкції
Кількість BeneFIX на флакон | Кількість BeneFIX на 1 мл відновленого розчину для ін'єкції |
250 ОД | 50 ОД |
500 ОД | 100 ОД |
1000 ОД | 200 ОД |
1500 ОД | 300 ОД |
2000 ОД | 400 ОД |
3000 ОД | 600 ОД |
Вигляд продукту та вміст упаковки
BeneFIX постачається у вигляді порошку для ін'єкції у скляному флаконі та розчинника, який постачається у попередньо наповненої серингу.
Упаковка містить:
- флакон з BeneFIX 250, 500, 1000, 1500, 2000 або 3000 ОД порошку
- попередньо наповнена серинга з розчинником, 5 мл розчину хлориду натрію 0,234% для відновлення
- стерильний адаптер для відновлення
- стерильна система для інфузії
- дві ватні тампони з алкоголем
- пластір для шкіри
- газова подушка
Власник дозволу на маркетинг
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Брюссель
Бельгія
Виробник
Wyeth Farma S.A
Autovía del Norte.A-1, Km. 23. Desvío Algete, Km. 1, 28700 Сан-Себастьян-де-лос-Рейес, Мадрид
Іспанія
Ви можете звернутися за додатковою інформацією про цей лікарський засіб до місцевого представника власника дозволу на маркетинг.
Бельгія/Бельгія/Бельгія Люксембург/Люксембург Pfizer NV/SA Тел.: +32 (0)2 554 62 11 | Литва Pfizer Luxembourg SARL filialas Lietuvoje Тел.: +3705 2514000 |
Україна Пфіцер Україна, ТОВ Тел.: +380 44 490 5500 | Угорщина Pfizer Kft. Тел.: + 36 1 488 37 00 |
Чехія Pfizer, spol. s r.o. Тел.: +420 283 004 111 | Мальта Vivian Corporation Ltd. Тел.: +356 21344610 |
Хорватія Pfizer Croatia d.o.o. Тел.: + 385 1 3908 777 | Словенія Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Любляна Тел.: + 386 (0)1 52 11 400 |
Ірландія Pfizer Healthcare Ireland Unlimited Company Тел.: 1800 633 363 (безкоштовно) Тел.: +44 (0)1304 616161 | Словаччина Pfizer Luxembourg SARL, organizacná zložka Тел.: + 421 2 3355 5500 |
Ісландія Icepharma hf. Тел.: + 354 540 8000 | Фінляндія Pfizer Oy Тел.: +358 (0)9 430 040 |
Італія Pfizer S.r.l. Тел.: +39 06 33 18 21 | Швеція Pfizer AB Тел.: +46 (0)8 550 520 00 |
Кіпр Pfizer Елл?ς Α.Ε. (філія на Кіпрі) Тел.: +357 22817690 | |
Латвія Pfizer Luxembourg SARL filiale Latvija Тел.: +371 670 35 775 | |
Дата останнього перегляду цієї інструкції: 03/2025.
Детальна інформація про цей лікарський засіб доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu. Також існують посилання на інші веб-сайти про рідкісні захворювання та орфанні лікарські засоби.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1000 МОДіючі речовини: coagulation factor IXВиробник: Swedish Orphan Biovitrum Ab (Publ)Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 2000 МОДіючі речовини: coagulation factor IXВиробник: Swedish Orphan Biovitrum Ab (Publ)Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 250 МОДіючі речовини: coagulation factor IXВиробник: Swedish Orphan Biovitrum Ab (Publ)Потрібен рецепт
Аналоги БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Польща
Аналог БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Україна
Лікарі онлайн щодо БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на БЕНЕФІКС 2000 МО, ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ – за рішенням лікаря та згідно з місцевими правилами.














