ALTUVOCT 250 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar ALTUVOCT 250 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ALTUVOCT 250 UI polvo y disolvente para solución inyectable
ALTUVOCT 500 UI polvo y disolvente para solución inyectable
ALTUVOCT 750 UI polvo y disolvente para solución inyectable
ALTUVOCT 1 000 UI polvo y disolvente para solución inyectable
ALTUVOCT 2 000 UI polvo y disolvente para solución inyectable
ALTUVOCT 3 000 UI polvo y disolvente para solución inyectable
ALTUVOCT 4 000 UI polvo y disolvente para solución inyectable
efanesoctocog alfa (factor VIII de coagulación recombinante humano)
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es ALTUVOCT y para qué se utiliza
- Qué necesita saber antes de empezar a usar ALTUVOCT
- Cómo usar ALTUVOCT
- Posibles efectos adversos
- Conservación de ALTUVOCT
- Contenido del envase e información adicional
1. Qué es ALTUVOCT y para qué se utiliza
ALTUVOCT contiene el principio activo efanesoctocog alfa, una proteína de sustitución del factor VIII.
ALTUVOCT se utiliza para tratar y prevenir los episodios hemorrágicos en pacientes con hemofilia A (un trastorno hemorrágico hereditario causado por una deficiencia del factor VIII) y se puede usar en pacientes de todos los grupos de edad.
El factor VIII es una proteína presente de forma natural en el cuerpo y es necesaria para que la sangre forme coágulos y detener las hemorragias. En los pacientes con hemofilia A, el factor VIII está ausente o no funciona adecuadamente.
ALTUVOCT reemplaza este «factor VIII» deficiente o ausente. ALTUVOCT aumenta las concentraciones de factor VIII en la sangre, ayudando así a la sangre a formar coágulos en el lugar de hemorragia, lo cual corrige temporalmente la tendencia a sufrir hemorragias.
2. Qué necesita saber antes de empezar a usar ALTUVOCT
No use ALTUVOCT
- si es alérgico a efanesoctocog alfa o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar ALTUVOCT.
- Existe una rara posibilidad de que sufra una reacción anafiláctica (una reacción alérgica grave y repentina) a ALTUVOCT. Entre los signos de las reacciones alérgicas se encuentran picor generalizado, ronchas, sensación de opresión en el pecho, dificultad para respirar y presión arterial baja. Si aparece cualquiera de estos síntomas, interrumpa inmediatamente la inyección y póngase en contacto con su médico.
- Consulte a su médico si cree que no está consiguiendo controlar sus hemorragias o las hemorragias de su hijo con la dosis que recibe, ya que pueden existir varios motivos para ello. En algunas personas que usan este medicamento se pueden desarrollar anticuerpos contra el factor VIII (también conocidos como inhibidores del factor VIII). La formación de inhibidores del factor VIII es una complicación conocida que puede ocurrir durante el tratamiento con todos los medicamentos de factor VIII. Estos inhibidores, especialmente a niveles altos, impiden que el tratamiento actúe adecuadamente; se vigilará atentamente en usted o en su hijo el desarrollo de estos inhibidores.
Acontecimientos cardiovasculares
Si tiene una enfermedad del corazón o corre el riesgo de padecerla, tenga especial cuidado cuando utilice medicamentos de factor VIII y consulte a su médico.
Complicaciones asociadas al catéter
Si necesita un dispositivo de acceso venoso central (DAVC), se debe tener en cuenta el riesgo de complicaciones relacionadas con el DAVC, incluidas las infecciones locales, la presencia de bacterias en la sangre y la trombosis en el lugar de inserción del catéter.
Otros medicamentos y ALTUVOCT
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
La influencia de ALTUVOCT sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
3. Cómo usar ALTUVOCT
El tratamiento con ALTUVOCT lo iniciará un médico con experiencia en el cuidado de pacientes con hemofilia A. ALTUVOCT se administra mediante inyección en una vena.
Después de recibir el entrenamiento necesario en la técnica correcta de inyección, los pacientes o cuidadores pueden administrar ALTUVOCT en el domicilio. Su médico calculará su dosis (en unidades internacionales [UI]). Esta dependerá de su peso y de si se usa para la prevención o para el tratamiento de las hemorragias.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Lleve un registro
Cada vez que use ALTUVOCT, anote la fecha, el nombre del medicamento y el número de lote.
Prevención de las hemorragias
La dosis habitual de ALTUVOCT es de 50 unidades internacionales (UI) por kilogramo de peso corporal. La inyección se administra una vez por semana.
Tratamiento de las hemorragias
La dosis de ALTUVOCT es de 50 unidades internacionales (UI) por kilogramo de peso corporal. La dosis y la frecuencia se pueden ajustar en función de la gravedad y de la localización de la hemorragia.
Uso en niños y adolescentes
ALTUVOCT se puede utilizar en niños de todas las edades; la recomendación relativa a la dosis es la misma que en los adultos.
Cómo se administra ALTUVOCT
ALTUVOCT se administra mediante inyección en una vena. Consulte «Instrucciones acerca de cómo usar ALTUVOCT» para más información.
Si usa más ALTUVOCT del que debe
Informe a su médico lo antes posible. Siga exactamente las instrucciones de administración de ALTUVOCT indicadas por su médico. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Si olvidó usar ALTUVOCT
No inyecte una dosis doble para compensar las dosis olvidadas. Inyecte su dosis tan pronto se acuerde y después reanude su pauta normal de dosificación. Si no está seguro de lo que debe hacer, consulte a su médico, farmacéutico o enfermero.
Si interrumpe el tratamiento con ALTUVOCT
Si interrumpe el tratamiento con ALTUVOCT, es posible que ya no esté protegido contra las hemorragias o que una hemorragia ya existente no se detenga. No interrumpa el tratamiento con ALTUVOCT sin consultar a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si se produce una reacción anafiláctica, la inyección se debe interrumpir inmediatamente y debe ponerse contacto inmediatamente con su médico.
Los síntomas de una reacción anafiláctica son, entre otros, los siguientes:
|
|
Riesgo de formación de inhibidores
En los niños que no han recibido tratamiento previo con medicamentos de factor VIII la formación de anticuerpos inhibidores (ver sección 2) es muy frecuente (pueden afectar a más de 1 de cada 10 pacientes); sin embargo, en los pacientes que han recibido tratamiento previo con factor VIII (más de 150 días de tratamiento) el riesgo es poco frecuente ((pueden afectar a hasta 1 de cada 100 pacientes). Si usted o su hijo experimentan la formación de anticuerpos inhibidores, el medicamento puede dejar de actuar adecuadamente y usted o su hijo pueden presentar hemorragia persistente. Si esto ocurre, debe ponerse en contacto con su médico inmediatamente.
Con este medicamento pueden aparecer los siguientes efectos adversos.
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- dolor de cabeza
- artralgia (dolor articular)
Efectos adversos frecuentes (pueden afectar a hasta 1 de cada 10 personas)
- dolor en las extremidades (brazos, manos, piernas o pies)
- dolor de espalda
- eccema (picor, enrojecimiento o sequedad de la piel)
- erupción cutánea
- urticaria (erupción con picor)
- fiebre
- vómitos
Efectos adversos poco frecuentes (pueden afectar a hasta 1 de cada 100 personas)
- reacciones en el lugar de inyección (incluyendo hematomas e inflamación)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ALTUVOCT
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el vial después de «CAD/EXP». La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC).
No congelar.
Conservar el vial en el embalaje exterior para protegerlo de la luz.
Antes de su reconstitución, el polvo de ALTUVOCT se puede conservar a temperatura ambiente (≤ 30 ºC) durante un único periodo no superior a 6 meses. Se debe anotar en la caja la fecha de extracción del medicamento de la nevera. Tras la conservación a temperatura ambiente, el medicamento no se debe volver a introducir en la nevera.
El medicamento no se debe utilizar después de la fecha de caducidad impresa en el vial o seis meses después de retirar la caja de la nevera, según cuál de estas circunstancias se produzca primero.
Una vez haya disuelto el polvo de ALTUVOCT en el disolvente suministrado en la jeringa precargada, debe utilizarlo inmediatamente. No refrigere la solución preparada.
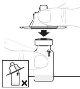
Tras la reconstitución, la solución debe ser transparente y entre incolora y ligeramente opalescente. No utilice este medicamento si observa que está turbio o contiene partículas visibles.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ALTUVOCT
- El principio activo es efanesoctocog alfa (factor VIII de coagulación recombinante humano).
Cada vial de ALTUVOCT contiene nominalmente 250, 500, 750, 1 000, 2 000, 3 000 o 4 000 UI de efanesoctocog alfa.
- Los demás componentes son sacarosa, cloruro de calcio dihidrato, histidina, hidrocloruro de arginina y polisorbato 80.
Aspecto del producto y contenido del envase
ALTUVOCT se presenta en forma de polvo y disolvente para solución inyectable. El polvo es un polvo suelto o compacto de color de blanco a blanquecino. El disolvente suministrado para la preparación de la solución inyectable es una solución transparente e incolora. Tras la preparación, la solución inyectable es transparente y entre incolora y ligeramente opalescente.
Cada envase de ALTUVOCT contiene 1 vial de polvo, 3 ml de disolvente en una jeringa precargada, 1 vástago del émbolo, 1 adaptador del vial y 1 equipo de perfusión.
Titular de la autorización de comercialización y responsable de la fabricación
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Stockholm
Suecia
Swedish Orphan Biovitrum AB (publ)
Norra Stationsgatan 93
113 64 Stockholm
Suecia
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Dé la vuelta al prospecto para ver instrucciones para la preparación y administración del medicamento.
Instrucciones acerca de cómo usar ALTUVOCT
LEA ATENTAMENTE ESTAS INSTRUCCIONES ANTES DE USAR ALTUVOCT
ALTUVOCT se administra mediante inyección intravenosa después de disolver el polvo inyectable con el disolvente suministrado en la jeringa precargada.
Si su dosis requiere más de un vial, recibirá varios envases e, idealmente, una jeringa grande.
Su profesional sanitario debe enseñarle a preparar e inyectar correctamente ALTUVOCT antes de que lo use por primera vez. Pregunte a su profesional sanitario si tiene alguna duda.
Información importante
Compruebe que el nombre y la dosis del medicamento son correctos y que conoce la frecuencia de administración de ALTUVOCT.
No utilice el medicamento si ha caducado, se ha abierto o parece estar dañado.
ALTUVOCT no se debe mezclar con otras soluciones inyectables.
Idealmente, ALTUVOCT se debe conservar en la nevera. Deje que el vial y la jeringa de disolvente alcancen la temperatura ambiente antes del uso. No utilice calor externo.
Compruebe que ninguno de los elementos presenta daños antes de su uso; no los utilice si parecen estar dañados.
Todos los elementos son válidos para un solo uso.
Lávese las manos y limpie una superficie plana antes de preparar el kit. Coloque la jeringa de forma segura sobre una superficie plana cuando no la esté manipulando.
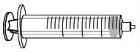
Guía de los elementos (incluidos en la caja)
ALTUVOCT se reconstituye disolviendo el polvo inyectable (A) en el disolvente suministrado en la jeringa precargada (B). Posteriormente, la solución de ALTUVOCT se debe administrar utilizando el equipo de perfusión (E).
|
|
|
|
|
polvo |
(precargada con disolvente) |
émbolo |
del vial |
perfusión |
Elementos adicionales (no incluidos en la caja)
Asegúrese de que dispone de toallitas con alcohol (F).
Es posible que su farmacéutico le haya suministrado una jeringa grande aparte (G) para extraer la solución de varios viales a una única jeringa. Si NO se le ha suministrado una jeringa grande, siga los pasos del 6 al 8 para administrar la solución de cada jeringa.
|
|
|
|
Reconstitución
| |
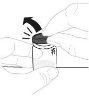
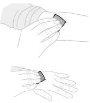
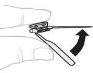
Sujete el vial de polvo (A) sobre una superficie limpia y plana y retire la cápsula de cierre de plástico. |
|
Limpie la parte superior del vial con una toallita con alcohol. Asegúrese de que nada entre en contacto con la parte superior del vial una vez que la haya limpiado. |
|
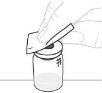
Desprenda la tapa protectora de papel del envase del adaptador del vial (D). No toque el adaptador del vial ni lo extraiga de su envase. |
|
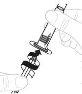
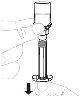
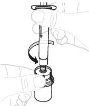
Coloque el envase del adaptador del vial directamente sobre la parte superior del vial. Presione firmemente hacia abajo hasta que el adaptador encaje en su posición. El perforador atravesará el tapón del vial. |
|
| |
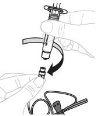
Inserte el vástago del émbolo (C) en la jeringa de 3 ml (B). Gire el vástago del émbolo en el sentido de las agujas del reloj hasta que quede bien acoplado. |
|
Separe la parte superior de la cápsula de cierre de la jeringa de 3 ml por las perforaciones y déjela a un lado. No toque el interior de la cápsula de cierre ni la punta de la jeringa. |
|
| |
Eleve el envase para separarlo del adaptador del vial y deséchelo. |
|
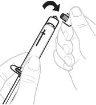
Sujete el adaptador del vial por el extremo inferior. Coloque la punta de la jeringa sobre la parte superior del adaptador del vial. Gire la jeringa en el sentido de las agujas del reloj hasta que quede bien acoplada. |
|
| |
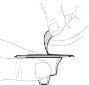
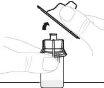
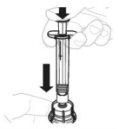
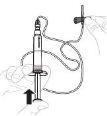
Presione lentamente el vástago del émbolo para inyectar todo el disolvente en el vial. |
|
Con el pulgar sobre el vástago del émbolo, mueva suavemente en círculos el vial hasta que el polvo se haya disuelto. No lo agite. |
|
Examine la solución antes de su administración. Debe ser transparente e incolora. No utilice la solución si está turbia o contiene partículas visibles. | |
| |
Si su dosis requiere más de un vial, siga los pasos indicados a continuación (5a y 5b); de lo contrario, siga en el paso 6. | |
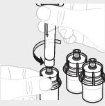
Repita los pasos del 1 al 4 con todos los viales hasta que haya preparado una cantidad suficiente de solución para su dosis. Retire las jeringas de 3 ml de cada vial (ver paso 6b), dejando la solución en cada vial. |
|
Para cada vial, acople la jeringa grande (G) al adaptador del vial (ver paso 3b) y realice el paso 6 para combinar la solución de cada vial en la jeringa grande. Si solo necesita parte de un vial entero, use la escala de graduación de la jeringa para ver la cantidad de solución que extrae , , tal como le haya indicado su profesional sanitario. |
|
| |
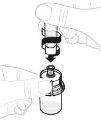
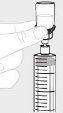
Coloque la jeringa apuntando hacia arriba. Tire lentamente del vástago del émbolo para trasladar toda la solución al interior de la jeringa. |
|
Desacople la jeringa del vial sujetando el adaptador del vial. Gire la jeringa en sentido contrario al de las agujas del reloj para desacoplarla. |
|
Administración
| |
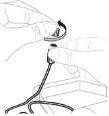
Abra el envase del equipo de perfusión (E) (no lo utilice si está dañado). Retire la cápsula de cierre del tubo. No toque el extremo expuesto del tubo. |
|
Acople la jeringa preparada al extremo del tubo del equipo de perfusión girando la jeringa en el sentido de las agujas del reloj. |
|
Si es necesario, aplique un torniquete. Limpie el lugar de inyección con una toallita con alcohol (F). |
|
Extraiga el aire colocando la jeringa apuntando hacia arriba y presionando suavemente el vástago del émbolo. No empuje la solución a través de la aguja. La inyección de aire en la vena puede ser peligrosa. |
|
| |
Retire la cubierta protectora de la aguja. Inserte la aguja en una vena, tal como le ha indicado su médico o enfermero, y retire el torniquete si lo ha aplicado. Puede usar una tirita para sujetar las alas de plástico de la aguja en su posición en el lugar de inyección para evitar que se mueva. | |
La solución preparada se debe inyectar por vía intravenosa entre 1 y 10 minutos en función del grado de comodidad del paciente. | |
| |
Retire la aguja. Pliegue el protector de la aguja; debería encajar en su posición. |
|
Deseche de forma segura la aguja usada, todo resto de solución no utilizado, la jeringa y el vial vacío en un contenedor de residuos médicos apropiado. No reutilice el instrumental. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ALTUVOCT 250 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 1.000 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1.500 UIPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 1000 UI - tras reconstitución en 2 ml de agua para inyectables la dosis es de 500 UI/mlPrincipio activo: factor de coagulación VIIIFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para ALTUVOCT 250 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ALTUVOCT 250 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes