АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Запитайте лікаря про рецепт на АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ

Інструкція із застосування АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Введення
Опис: інформація для користувача
ALTUVOCT 250 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 500 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 750 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 1 000 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 2 000 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 3 000 МО порошок і розчинник для ін'єкційного розчину
ALTUVOCT 4 000 МО порошок і розчинник для ін'єкційного розчину
ефанесоктоког альфа (рекомбінантний людський фактор згортання VIII)
Цей лікарський засіб підлягає додатковому моніторингу, що полегшить виявлення нової інформації про його безпеку. Ви можете допомогти, повідомляючи про будь-які побічні ефекти, які ви можете мати. Остання частина розділу 4 містить інформацію про те, як повідомляти про ці побічні ефекти.
Прочитайте уважно весь опис перед тим, як почати використовувати цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
- Цей лікарський засіб призначений тільки вам, і не давайте його іншим людям, навіть якщо вони мають такі самі симптоми, як у вас, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому описі. Див. розділ 4.
Зміст опису
- Що таке ALTUVOCT і для чого він використовується
- Що вам потрібно знати перед тим, як почати використовувати ALTUVOCT
- Як використовувати ALTUVOCT
- Можливі побічні ефекти
- Зберігання ALTUVOCT
- Зміст упаковки та додаткова інформація
1. Що таке ALTUVOCT і для чого він використовується
ALTUVOCT містить активну речовину ефанесоктоког альфа, білок-замінник фактору згортання VIII.
ALTUVOCT використовується для лікування та профілактики геморагічних епізодів у пацієнтів з гемофілією А (геморагічний спадковий розлад, викликаний дефіцитом фактору VIII) і може бути використаний у пацієнтів усіх вікових груп.
Фактор VIII - це білок, який природно присутній у організмі і необхідний для того, щоб кров утворювала згустки і зупиняла кровотечі. У пацієнтів з гемофілією А фактор VIII відсутній або не функціонує належним чином.
ALTUVOCT заміняє цей "фактор VIII", який відсутній або дефектний. ALTUVOCT збільшує концентрацію фактору VIII у крові, що допомагає крові утворювати згустки в місці кровотечі, тим самим тимчасово виправляючи схильність до кровотеч.
2. Що вам потрібно знати перед тим, як почати використовувати ALTUVOCT
Не використовуйте ALTUVOCT
- якщо ви алергічні на ефанесоктоког альфа або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6).
Попередження та обережність
Проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою перед тим, як почати використовувати ALTUVOCT.
- Існує рідка можливість того, що ви можете мати анафілактичну реакцію (тяжку і раптову алергічну реакцію) на ALTUVOCT. Серед ознак алергічних реакцій - загальне свербіння, висип, відчуття стискання в грудній клітці, труднощі з диханням і низький кров'яний тиск. Якщо з'являються будь-які з цих симптомів, негайно припиніть ін'єкцію і зверніться до вашого лікаря.
- Проконсультуйтеся з вашим лікарем, якщо ви вважаєте, що не можете контролювати кровотечі або кровотечі вашої дитини за допомогою дози, яку ви отримуєте, оскільки можуть бути різні причини цього. У деяких людей, які використовують цей лікарський засіб, можуть розвиватися антитіла проти фактору VIII (також відомі як інгібітори фактору VIII). Формування інгібіторів фактору VIII - це відома ускладнення, яка може виникнути під час лікування всіма лікарськими засобами фактору VIII. Ці інгібітори, особливо на високих рівнях, перешкоджають належній дії лікування; буде проводитися ретельний моніторинг у вас або вашої дитини щодо розвитку цих інгібіторів.
Серцево-судинні події
Якщо у вас є захворювання серця або ви перебуваєте в групі ризику серцевих захворювань, будьте особливо обережні при використанні лікарських засобів фактору VIII і проконсультуйтеся з вашим лікарем.
Ускладнення, пов'язані з катетером
Якщо вам потрібно центральний венозний катетер, потрібно враховувати ризик ускладнень, пов'язаних з катетером, включаючи місцеві інфекції, бактеріємію та тромбоз в місці введення катетера.
Інші лікарські засоби та ALTUVOCT
Повідоміть вашого лікаря, якщо ви використовуєте, нещодавно використовували або можете використовувати будь-який інший лікарський засіб.
Вагітність і лактація
Якщо ви вагітні або перебуваєте в період лактації, вважаєте, що можете бути вагітні або плануєте вагітність, проконсультуйтеся з вашим лікарем перед тим, як використовувати цей лікарський засіб.
Водіння транспортних засобів і використання машин
Вплив ALTUVOCT на здатність водити транспортні засоби та використовувати машини є незначним або відсутнім.
3. Як використовувати ALTUVOCT
Лікування ALTUVOCT буде розпочато лікарем, який має досвід у лікуванні пацієнтів з гемофілією А. ALTUVOCT вводиться шляхом ін'єкції в вену.
Після отримання необхідної підготовки щодо техніки ін'єкції пацієнти або опікуни можуть вводити ALTUVOCT вдома. Ваш лікар розрахує вашу дозу (у міжнародних одиницях [МО]). Вона залежить від вашої ваги та того, чи використовується вона для профілактики чи лікування кровотеч.
Слідуйте точно інструкціям щодо введення цього лікарського засобу, вказаним вашим лікарем. У разі сумнівів проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
Ведіть реєстр
Кожного разу, коли ви використовуєте ALTUVOCT, записуйте дату, назву лікарського засобу та номер партії.
Профілактика кровотеч
Звичайна доза ALTUVOCT становить 50 міжнародних одиниць (МО) на кілограм ваги. Ін'єкція вводиться один раз на тиждень.
Лікування кровотеч
Доза ALTUVOCT становить 50 міжнародних одиниць (МО) на кілограм ваги. Доза та частота можуть бути调整 залежно від тяжкості та місця кровотечі.
Використання у дітей та підлітків
ALTUVOCT можна використовувати у дітей усіх вікових груп; рекомендація щодо дози така сама, як у дорослих.
Як вводити ALTUVOCT
ALTUVOCT вводиться шляхом ін'єкції в вену. Див. «Інструкції щодо використання ALTUVOCT» для отримання додаткової інформації.
Якщо ви використовуєте більше ALTUVOCT, ніж потрібно
Повідоміть вашого лікаря якомога швидше. Слідуйте точно інструкціям щодо введення ALTUVOCT, вказаним вашим лікарем. У разі сумнівів проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
Якщо ви забули використати ALTUVOCT
Не вводьте подвійну дозу для компенсації забутих доз. Вводьте вашу дозу якомога швидше, коли ви про це пам'ятайте, і потім поверніться до вашої звичайної схеми дозування. Якщо ви не впевнені в тому, що вам потрібно зробити, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою.
Якщо ви припиняєте лікування ALTUVOCT
Якщо ви припиняєте лікування ALTUVOCT, ви можете перестати бути захищеними від кровотеч або кровотеча, яка вже існує, не зупиниться. Не припиняйте лікування ALTUVOCT без консультації з вашим лікарем.
Якщо у вас є якісь інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Якщо відбувається анафілактична реакція, ін'єкцію потрібно негайно припинити і звернутися до вашого лікаря.
Симптоми анафілактичної реакції включають:
|
|
Ризик формування інгібіторів
У дітей, які не мали попереднього лікування лікарськими засобами фактору VIII, формування інгібіторів (див. розділ 2) дуже часто зустрічається (може вплинути на понад 1 з 10 пацієнтів); однак у пацієнтів, які мали попереднє лікування фактором VIII (більше 150 днів лікування), ризик є рідкісним (може вплинути на до 1 з 100 пацієнтів). Якщо ви або ваша дитина відчуваєте формування інгібіторів, лікарський засіб може перестати діяти належним чином, і ви або ваша дитина можете мати тривалу кровотечу. Якщо це відбувається, вам потрібно негайно звернутися до вашого лікаря.
З цим лікарським засобом можуть виникнути наступні побічні ефекти.
Дуже часті побічні ефекти (можуть вплинути на понад 1 з 10 осіб)
- головний біль
- артралгія (біль у суглобах)
Часті побічні ефекти (можуть вплинути на до 1 з 10 осіб)
- біль у кінцівках (руках, ногах, ступнях або руках)
- біль у спині
- екзема (свербіння, червоність або сухість шкіри)
- висип
- кропив'янка (висип із свербінням)
- лихоманка
- вомітування
Рідкісні побічні ефекти (можуть вплинути на до 1 з 100 осіб)
- реакції в місці ін'єкції (включаючи гематоми та запалення)
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з вашим лікарем, фармацевтом або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому описі. Ви також можете повідомити про них безпосередньо через національну систему повідомлення, вказану в додатку V. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання ALTUVOCT
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте цей лікарський засіб після закінчення терміну його дії, вказаного на коробці та флаконі після «CAD/EXP». Термін дії - останній день місяця, який вказано.
Тримайте в холодильнику (між 2 °C і 8 °C).
Не заморожуйте.
Тримайте флакон у зовнішній упаковці, щоб захистити його від світла.
До його реконституції порошок ALTUVOCT можна зберігати при кімнатній температурі (≤ 30 °C) протягом одного періоду, який не перевищує 6 місяців. На коробці потрібно вказати дату видалення лікарського засобу з холодильника. Після зберігання при кімнатній температурі лікарський засіб не повинен бути повернутий до холодильника.
Лікарський засіб не повинен бути використаний після закінчення терміну його дії, вказаного на флаконі, або через 6 місяців після видалення коробки з холодильника, залежно від того, яке з цих обставин відбувається раніше.
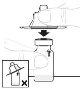
Після розчинення порошку ALTUVOCT у розчиннику, який поставляється з попередньо заповненою шприцем, його потрібно використовувати негайно. Не зберігайте приготований розчин у холодильнику.
Після реконституції розчин повинен бути прозорим і безколірним або легенько опалесцентним. Не використовуйте цей лікарський засіб, якщо ви помітите, що він є мутним або містить видимі частинки.
Лікарські засоби не повинні бути викинуті у каналізацію або сміття. Спитайте у вашого фармацевта, як позбутися упаковок та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад АLTUVOCT
- Активний інгредієнт - ефанесоктоког альфа (рекомбінантний людський фактор згортання крові VIII).
Кожна флакона АLTUVOCT містить номінально 250, 500, 750, 1 000, 2 000, 3 000 або 4 000 ОД ефанесоктоку альфа.
- Інші компоненти - сукроза, дигідрат дікальцієвого хлориду, гістидин, аргінін гідрохлорид та полісорбат 80.
Вигляд продукту та вміст упаковки
АLTUVOCT випускається у вигляді порошку та розчинника для ін'єкційної розчини. Порошок - це вільний або пресований порошок білого або білуватого кольору. Розчинник, який постачається для підготовки ін'єкційної розчини, - це прозора та безбарвна рідина. Після підготовки ін'єкційна розчина прозора та безбарвна або легка опалесценція.
Кожна упаковка АLTUVOCT містить 1 флакон порошку, 3 мл розчинника у попередньо заповненому шприці, 1 поршень шприця, 1 адаптер флакона та 1 набір для інфузії.
Уповноважений на отримання дозволу на продаж та відповідальний за виробництво
Swedish Orphan Biovitrum AB (publ)
SE-112 76 Стокгольм
Швеція
Swedish Orphan Biovitrum AB (publ)
Норра Стансгатан 93
113 64 Стокгольм
Швеція
Дата останнього перегляду цієї інструкції:
Детальна інформація про цей препарат доступна на сайті Європейського агентства з лікарських засобів: https://www.ema.europa.eu.
Переверніть інструкцію, щоб побачити інструкції щодо підготовки та введення препарату.
Інструкції щодо використання АLTUVOCT
ВВАЖНО ПРОЧИТАЙТЕ ЦІ ІНСТРУКЦІЇ ПЕРЕД ВЖИТТЯМ АLTUVOCT
АLTUVOCT вводиться шляхом внутрішньовенної ін'єкції після розчинення порошку ін'єкційної розчини з розчинником, який постачається у попередньо заповненому шприці.
Якщо ваша доза вимагає більше одного флакона, ви отримаєте кілька упаковок та, ідеально, великий шприц.
Ваш лікар повинен навчити вас правильно підготувати та ввести АLTUVOCT перед тим, як ви його вперше використаєте. Зверніться до свого лікаря, якщо у вас є якісь питання.
Важлива інформація
Перевірте, чи правильна назва та доза препарату, та чи знаєте ви частоту введення АLTUVOCT.
Не використовуйте препарат, якщо він просрочений, відкритий або здається пошкодженим.
АLTUVOCT не слід змішувати з іншими ін'єкційними розчинами.
Ідеально, АLTUVOCT повинен зберігатися у холодильнику. Дайте флакону та шприцю розчинника досягти кімнатної температури перед використанням. Не використовуйте зовнішнє тепло.
Перевірте, чи жоден з елементів не пошкоджений перед використанням; не використовуйте їх, якщо вони здаються пошкодженими.
Всі елементи призначені для одного разового використання.
Вимийте руки та очистите плоску поверхню перед підготовкою набору. Помістіть шприц у безпечному місці на плоскій поверхні, коли не маніпулюєте ним.
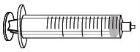
Посібник елементів (включених до коробки)
АLTUVOCT реконститується шляхом розчинення порошку ін'єкційної розчини (А) у розчиннику, який постачається у попередньо заповненому шприці (Б). Після цього розчин АLTUVOCT слід вводити за допомогою набору для інфузії (Е).
|
|
|
|
|
порошком |
(попередньо заповнений розчинником) |
шприця |
флакона |
інфузії |
Додаткові елементи (не включені до коробки)
Перевірте, чи маєте ви під рукою ватні диски з алкоголем (Ф).
Можливо, ваш фармацевт надав вам великий шприц окремо (Г) для видалення розчину з кількох флаконів до одного шприця. Якщо ВАМ не надали великий шприц, слідуйте крокам з 6 до 8 для введення розчину з кожного шприця.
|
|
|
|
Реконституція
| |
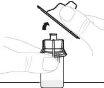
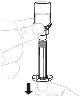
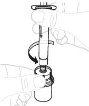
Утримуйте флакон з порошком (А) над чистою та плоскою поверхнею та видаліть пластикову кришку. |
|
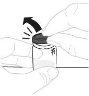
Очистите верхню частину флакона ватним диском з алкоголем. Перевірте, чи нічого не торкається верхньої частини флакона після його очистки. |
|
Відкрийте паперову кришку упаковки адаптера флакона (Д). Не торкайтеся адаптера флакона та не видаліть його з упаковки. |
|
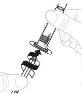
Помістіть упаковку адаптера флакона безпосередньо на верхню частину флакона. Тисніть вниз сильно, поки адаптер не займе своє місце. Перфоратор пройде через кришку флакона. |
|
| |
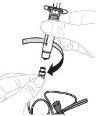
Вставьте поршень шприця (С) до шприця 3 мл (Б). Повертіть поршень шприця за годинниковою стрілкою, поки він не буде добре прикріплений. |
|
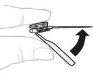
Відокремте верхню частину кришки шприця 3 мл по перфорації та покладіть її вбок. Не торкайтеся внутрішньої частини кришки чи кінця шприця. |
|
| |
Підніміть упаковку, щоб відокремити її від адаптера флакона, та викиньте її. |
|
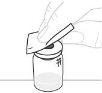
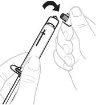
Утримуйте адаптер флакона за нижній кінець. Помістіть кінець шприця на верхню частину адаптера флакона. Повертіть шприц за годинниковою стрілкою, поки він не буде добре прикріплений. |
|
| |
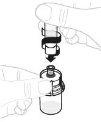
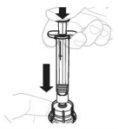
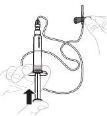
Тисніть повільно поршень шприця, щоб ввести весь розчинник до флакона. |
|
З великим пальцем на поршні шприця, рухайте флакон м'яко по колу, поки порошок не розчиниться. Не агітуйте. |
|
Перевірте розчин перед введенням. Він повинен бути прозорим та безбарвним. Не використовуйте розчин, якщо він мутний або містить видимі частинки. | |
| |
Якщо ваша доза вимагає більше одного флакона, слідуйте крокам, вказаним нижче (5а та 5б); інакше перейдіть до кроку 6. | |
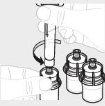
Повторіть кроки з 1 до 4 для всіх флаконів, поки не підготуєте достатню кількість розчину для вашої дози. Видаліть шприці 3 мл з кожного флакона (див. крок 6б), залишивши розчин у кожному флаконі. |
|
Для кожного флакона прикріпіть великий шприц (Г) до адаптера флакона (див. крок 3б) та виконайте крок 6, щоб об'єднати розчин з кожного флакона до великого шприця. Якщо вам потрібно лише частина цілого флакона, використовуйте градуйовану шкалу шприця, щоб побачити кількість розчину, який ви видаляєте, як вказав ваш лікар. |
|
| |
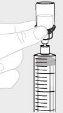
Помістіть шприц вверх. Тисніть повільно поршень шприця, щоб перемістити весь розчин до шприця. |
|
Від'єднайте шприц від флакона, утримуючи адаптер флакона. Повертіть шприц проти годинникової стрілки, щоб від'єднати його. |
|
Введення
| |
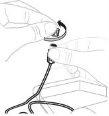
Відкрийте упаковку набору для інфузії (Е) (не використовуйте, якщо він пошкоджений). Видаліть кришку трубки. Не торкайтеся відкритого кінця трубки. |
|
Прикріпіть підготовлений шприц до кінця трубки набору для інфузії, повертаючи шприц за годинниковою стрілкою. |
|
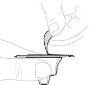
Якщо необхідно, застосуйте джгут. Очистите місце ін'єкції ватним диском з алкоголем (Ф). |
|
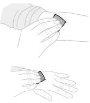
Видаліть повітря, помістивши шприц вверх та тиснучи м'яко поршень шприця. Не штовхайте розчин через голку. Введення повітря в вену може бути небезпечним. |
|
| |
Видаліть захисний ковпачок голки. Вставьте голку до вени так, як вказав ваш лікар або медсестра, та видаліть джгут, якщо ви його застосували. Ви можете використовувати тирку, щоб утримувати пластикові крила голки на місці ін'єкції, щоб уникнути руху. | |
Підготовлений розчин слід вводити внутрішньовенно протягом 1-10 хвилин, залежно від ступеня комфорту пацієнта. | |
| |
Видаліть голку. Згиніть захисний ковпачок голки; він повинен зайняти своє місце. |
|
Видаліть використану голку, будь-який залишковий розчин, шприц та порожній флакон у відповідний контейнер для медичних відходів. Не повторно використовуйте інструменти. |
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1000 МОДіючі речовини: coagulation factor VIIIВиробник: Takeda Manufacturing Austria AgПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1500 МОДіючі речовини: coagulation factor VIIIВиробник: Takeda Manufacturing Austria AgПотрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 1000 МО - після відновлення в 2 мл води для ін'єкцій доза становить 500 МО/млДіючі речовини: coagulation factor VIIIВиробник: Takeda Manufacturing Austria AgПотрібен рецепт
Аналоги АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Польща
Аналог АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Україна
Лікарі онлайн щодо АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на АЛТУВОКТ 2000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ – за рішенням лікаря та згідно з місцевими правилами.