ZYPADHERA 300 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA

Cómo usar ZYPADHERA 300 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ZYPADHERA 210 mg polvo y disolvente para suspensión inyectable de liberación prolongada
ZYPADHERA 300 mg polvo y disolvente para suspensión inyectable de liberación prolongada
ZYPADHERA 405 mg polvo y disolvente para suspensión inyectable de liberación prolongada
Olanzapina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es ZYPADHERA y para qué se utiliza
- Qué necesita saber antes de empezar a usar ZYPADHERA
- Cómo usar ZYPADHERA
- Posibles efectos adversos
- Conservación de ZYPADHERA
- Contenido del envase e información adicional
1. Qué es ZYPADHERA y para qué se utiliza
ZYPADHERA contiene olanzapina como sustancia activa. ZYPADHERA pertenece a un grupo de medicamentos denominados antipsicóticos y se utiliza para tratar la esquizofrenia – una enfermedad con síntomas tales como oír, ver o percibir cosas que no existen, creencias erróneas, suspicacia inusual y retraimiento. Las personas con esta enfermedad también pueden sentirse deprimidas, ansiosas o tensas.
ZYPADHERA está indicado en pacientes adultos que han sido estabilizados previamente durante el tratamiento con olanzapina oral.
2. Qué necesita saber antes de empezar a usar ZYPADHERA
No use ZYPADHERA:
- si es alérgico a olanzapina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Una reacción alérgicapuede manifestarse en forma de erupción cutánea, picor, hinchazón de la cara, de los labios, o dificultad para respirar. Si ha sufrido alguno de estos síntomas alguna vez, por favor informe a su médico o enfermero.
- si previamente se le han diagnosticado problemas oculares, tales como ciertos tipos de glaucoma (aumento de presión en el ojo).
Advertencias y precauciones
Consulte a su médico o enfermero antes de empezar a usar ZYPADHERA.
- Una reacción poco frecuente pero grave, puede ocurrir después de que reciba cada inyección.A veces, ZYPADHERA puede entrar en el torrente sanguíneo demasiado rápido. Si esto le ocurriese, podría sufrir alguno de los síntomas enumerados a continuación después de cada inyección. En algunos casos, estos síntomas pueden provocar la pérdida del conocimiento.
• | excesiva somnolencia | • | mareo | |
• | confusión | • | desorientación | |
• | irritabilidad | • | ansiedad | |
• | agresividad |
| ||
• | dificultad para hablar | • | sanguínea | |
• | dificultad para caminar | debilidad | ||
• | convulsiones |
|
Estos síntomas normalmente desaparecen en 24 a 72 horas después de la inyección. Después de cada inyección deberá permanecer en observación en su centro sanitario durante al menos 3 horas por si presenta alguno de los síntomas arriba indicados.
Aunque sea poco probable, puede sufrir estos síntomas una vez transcurridas 3 horas después de la inyección. Si esto le ocurriese, póngase en contacto con su médico o enfermero inmediatamente. Como consecuencia de este riesgo, no debe conducir vehículos ni manejar máquinas durante el resto del día después de cada inyección.
- Si se siente mareado o se desmaya después de la inyección, informe a su médico o enfermero. Es probable que tenga que acostarse hasta que se sienta mejor. Puede que el médico o enfermero quiera tomarle la tensión y comprobar su pulso.
- No se recomienda el uso de ZYPADHERA en pacientes de edad avanzada con demencia(confusión o pérdida de memoria) ya que puede provocar efectos adversos graves.
- En muy raras ocasiones, los medicamentos de este tipo pueden causar movimientos inusuales, principalmente en la cara o la lengua o una combinación de fiebre, respiración acelerada, sudoración, rigidez muscular y sopor o somnolencia. Si esto le ocurriese después de recibir ZYPADHERA, informe a su médico o a su enfermero de forma inmediata.
- Se ha observado un aumento de peso en los pacientes que están tomando ZYPADHERA. Usted y su médico deben comprobar su peso con regularidad. Si fuera necesario, su médico le puede ayudar a planificar una dieta o considerar la posibilidad de remitirle a un nutricionista.
- Se han observado niveles elevados de azúcar y grasas (triglicéridos y colesterol) en sangre en los pacientes que están usando ZYPADHERA. Su médico debe hacerle análisis de sangre para controlar su azúcar en sangre y los niveles de grasa antes de que comience a usar ZYPADHERA y de forma regular durante el tratamiento.
- Informe a su médico si usted o alguien en su familia tiene antecedentes de coágulos sanguíneos, ya que los medicamentos de este tipo han sido asociados con la formación de coágulos en la sangre.
Informe a su médico lo antes posible si sufre alguna de las siguientes afecciones:
- Ictus o “mini” ictus (síntomas transitorios de accidente cerebrovascular)
- Enfermedad de Parkinson
- Problemas de próstata
- Obstrucción intestinal (Íleo paralítico)
- Enfermedad hepática o renal
- Trastornos de la sangre
- Un infarto reciente, enfermedad coronaria, síndrome del seno enfermo (ritmos cardíacos anormales), angina inestable o tensión arterial baja.
- Diabetes
- Convulsiones
- Si cree que puede tener pérdida de sales como consecuencia de tener diarrea y vómitos intensos de forma prolongada o por el uso de medicamentos diuréticos (comprimidos para orinar)
Como precaución rutinaria, se recomienda medir la tensión arterial periódicamente en pacientes mayores de 65 años.
No se recomienda comenzar el tratamiento con ZYPADHERA si usted es mayor de 75 años.
Niños y adolescentes
Los pacientes menores de 18 años no deben usar ZYPADHERA.
Otros medicamentos y ZYPADHERA
Informe a su médico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Especialmente, informe a su médico si está tomando:
- medicamentos para la enfermedad de Parkinson.
- carbamazepina (un antiepiléptico y estabilizador del humor), fluvoxamina (un antidepresivo) o ciprofloxacino (un antibiótico) – es posible que se tenga que modificar su dosis de
ZYPADHERA.
Si ya está tomando antidepresivos, medicamentos para aliviar la ansiedad o para ayudarle a dormir (tranquilizantes), puede sentirse más somnoliento si toma ZYPADHERA.
Uso de ZYPADHERA con alcohol
Debe evitar todo consumo de alcohol si se le ha administrado ZYPADHERA, ya que en combinación con alcohol, puede causar somnolencia.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de recibir esta inyección.
No debe recibir esta inyección si está dando el pecho, ya que pequeñas cantidades de olanzapina pueden pasar a la leche materna.
Se pueden producir los siguientes síntomas en bebés recién nacidos, de madres que han sido tratadas con ZYPADHERA en el último trimestre de embarazo (últimos tres meses de su embarazo): temblor, rigidez y/o debilidad muscular, somnolencia, agitación, problemas al respirar, y dificultad en la alimentación. Si su bebé desarrolla cualquiera de estos síntomas se debe poner en contacto con su médico.
Conducción y uso de máquinas
No conduzca ni utilice máquinas durante el resto del día después de cada inyección.
ZYPADHERA contiene sodio
Una vez reconstituido este medicamento contiene menos de 1 mmol de sodio (23 mg) por vial; esto es, esencialmente “exento de sodio”.
3. Cómo usar ZYPADHERA
Su médico decidirá la cantidad de ZYPADHERA que necesita y con qué frecuencia necesita recibir una inyección. ZYPADHERA se administra en dosis de 150 mg a 300 mg cada 2 semanas o de 300 mg a 405 mg cada 4 semanas.
ZYPADHERA se presenta en forma de polvo que su médico o enfermero reconstituirá para crear una suspensión que después le será inyectada en el músculo de la nalga.
Si usa más ZYPADHERA del que debe
Este medicamento se le administrará bajo supervisión médica. Por lo tanto, es poco probable que reciba una cantidad excesiva.
Los pacientes que han recibido más olanzapina de la que debían, también han experimentado los siguientes síntomas:
- ritmo cardíaco acelerado, agitación/agresividad, problemas de habla, movimientos inusuales (especialmente en la cara o la lengua) y niveles disminuidos de conciencia.
Otros síntomas podrían incluir:
- confusión aguda, convulsiones (epilepsia), coma, una combinación de fiebre, respiración acelerada, sudoración, entumecimiento muscular, y un estado de sopor o somnolencia, respiración más lenta, aspiración, tensión arterial elevada o baja, ritmos cardíacos anómalos.
Póngase en contacto con su médico u hospital de forma inmediata si experimenta cualquiera de los síntomas previamente descritos.
Si olvidó usar ZYPADHERA
No interrumpa su tratamiento solo porque empieza a sentirse mejor. Es importante que se le siga administrando ZYPADHERA durante todo el tiempo indicado por su médico.
Si falta a la cita para su inyección, debe ponerse en contacto con su médico para concertar la próxima inyección lo antes posible.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Póngase en contacto inmediatamente con su médico si usted tiene:
- somnolencia excesiva, mareos, confusión, desorientación, dificultad para hablar, dificultad para andar, rigidez muscular o agitación, debilidad, irritabilidad, agresividad, ansiedad, aumento de la presión sanguínea o convulsiones y puede llegar a provocar pérdida de conocimiento. Estos signos y síntomas pueden deberse a que a veces ZYPADHERA puede entrar en el torrente sanguíneo demasiado rápido (efecto adverso frecuente que puede afectar hasta 1 de cada 10 personas);
- movimientos inusuales (un efecto adverso frecuente que puede afectar hasta 1 de cada 10 personas) especialmente de la cara o de la lengua;
- coágulos sanguíneos en las venas (un efecto adverso poco frecuente que puede afectar hasta 1 de cada 100 personas), especialmente en las piernas (los síntomas incluyen hinchazón, dolor y enrojecimiento en la pierna), que pueden viajar a través de la sangre hacia los pulmones, causando dolor en el pecho y dificultad para respirar. Si experimenta cualquiera de estos síntomas, acuda al médico de inmediato.
- combinación de fiebre, respiración acelerada, sudoración, rigidez muscular y un estado de obnubilación o somnolencia (la frecuencia no puede ser estimada a partir de los datos disponibles)
Otros efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas) con ZYPADHERA incluyen somnolencia y dolor en el lugar de inyección.
Dentro de los efectos adversos raros con ZYPADHERA (pueden afectar a 1 de cada 1.000 personas) se incluye infección en el lugar de la inyección.
Los efectos adversos que se enumeran a continuación se han observado al administrar olanzapina por vía oral, pero pueden aparecer después de la administración de ZYPADHERA.
Otros efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 personas) incluyen aumento de peso y aumento de los niveles de prolactina en sangre. En las primeras fases del
tratamiento, algunas personas pueden sentir mareos o desmayos (con latidos del corazón más lentos), sobre todo al incorporarse cuando están tumbados o sentados. Esta sensación suele desaparecer espontáneamente, pero si no ocurriera así, consulte con su médico.
Otros efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 personas) incluyen cambios en los niveles de algunas células sanguíneas, grasas en el torrente sanguíneo y al comienzo del tratamiento aumentos temporales de las enzimas hepáticas; aumentos del nivel de azúcares en sangre y orina; aumento de los niveles de ácido úrico y creatinfosfoquinasa en sangre; aumento del apetito; mareos; agitación; temblor; movimientos extraños (discinesias); estreñimiento; sequedad de boca; erupción cutánea; pérdida de fuerza; cansancio exagerado; retención de líquidos produciendo hinchazón en las manos, tobillos o pies; fiebre; dolor en las articulaciones; y disfunciones sexuales tales como disminución de la líbido en hombres y mujeres o disfunción eréctil en hombres.
Otros efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 personas) incluyen hipersensibilidad (p. ej. inflamación de la boca y de la garganta, picores; erupción en la piel); diabetes o empeoramiento de la diabetes, relacionados ocasionalmente con cetoacidosis (acetona en sangre y orina) o coma; convulsiones, en la mayoría de los casos se relacionan con antecedentes de convulsiones (epilepsia); rigidez muscular o espasmos (incluyendo movimientos de los ojos); síndrome de piernas inquietas; problemas con el habla; tartamudeo; ritmo cardíaco lento; sensibilidad a la luz solar; sangrado por la nariz; distensión abdominal; salivación excesiva; pérdida de memoria u olvidos; incontinencia urinaria; pérdida de la habilidad para orinar; caída del pelo; ausencia o disminución de los períodos menstruales; y cambios en la glándula mamaria en hombres y en mujeres tales como producción anormal de leche materna o crecimiento anormal.
Efectos adversos raros (pueden afectar hasta 1 persona de cada 1000) incluyen disminución de la temperatura corporal normal; ritmos cardíacos anómalos; muerte súbita de origen desconocido; inflamación del páncreas que provoca dolor de estómago intenso; fiebre y vómitos; enfermedad hepática que se manifiesta como una coloración amarillenta de la piel y de la parte blanca de los ojos; enfermedad muscular que se manifiesta con dolores articulares de origen desconocido; y erección prolongada y/o dolorosa.
Efectos adversos muy raros incluyen reacciones alérgicas graves tales como reacción a fármaco con eosinofilia y síntomas sistémicos (DRESS por sus siglas en inglés). Inicialmente DRESS se manifiesta con síntomas similares a la gripe con sarpullido en la cara y posteriormente, con un sarpullido extenso, fiebre, ganglios linfáticos agrandados, elevación de enzimas hepáticas observado en análisis de sangre y aumento de un tipo de glóbulos blancos de la sangre (eosinofilia).
Durante el tratamiento con olanzapina, los pacientes de edad avanzada con demencia pueden sufrir ictus, neumonía, incontinencia urinaria, caídas, cansancio exagerado, alucinaciones visuales, aumento de la temperatura corporal, enrojecimiento de la piel y dificultad para caminar. Algunos casos de fallecimiento se han observado en este grupo de pacientes en particular.
Olanzapina por vía oral puede agravar los síntomas en pacientes con la enfermedad de Parkinson.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ZYPADHERA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No administrar la inyección después de la fecha de caducidad indicada en el envase.
No refrigerar ni congelar.
Se ha demostrado la estabilidad química y física de la suspensión en los viales durante 24 horas a 20 - 25ºC. Desde un punto de vista microbiológico, el medicamento debe administrarse inmediatamente. Si no es así, los tiempos de almacenamiento y las condiciones de uso antes de su empleo son responsabilidad del profesional sanitario y normalmente no debe superar las 24 horas a 20 - 25ºC. No use este producto si nota decoloración u otros signos visibles de deterioro.
Si no se utiliza el medicamento de forma inmediata, debe agitarse de forma vigorosa para conseguir la resuspensión. Una vez retirada la suspensión del vial a la jeringa, debe usarse de inmediato.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ZYPADHERA
El principio activoes olanzapina.
ZYPADHERA 210 mg: Cada vial contiene pamoato de olanzapina monohidratado, equivalente a 210 mg de olanzapina.
ZYPADHERA 300 mg: Cada vial contiene pamoato de olanzapina monohidratado, equivalente a 300 mg de olanzapina.
ZYPADHERA 405 mg: Cada vial contiene pamoato de olanzapina monohidratado, equivalente a 405 mg de olanzapina
Una vez reconstituida, cada mililitro de la suspensión contiene 150 mg/ml de olanzapina.
Los componentes del disolventeson carmelosa sódica, manitol, polisorbato 80, agua para preparaciones inyectables, ácido clorhídrico e hidróxido de sodio.
Aspecto del producto y contenido del envase
ZYPADHERA polvo para suspensión inyectable de liberación prolongada se presenta como un polvo amarillo en un vial de vidrio transparente. Su médico o enfermero lo reconstituirá en una suspensión que se administrará en forma de inyección utilizando el contenido del vial de disolvente para ZYPADHERA que se presenta como una solución transparente, incolora o de color amarillo pálido, dentro de un vial de vidrio transparente.
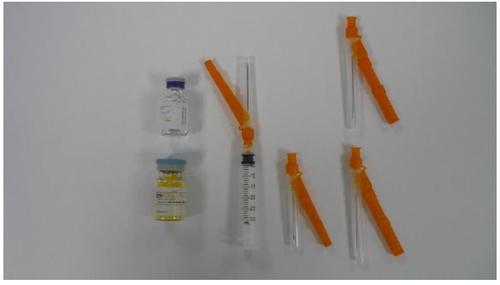
ZYPADHERA es un polvo y disolvente para suspensión inyectable de liberación prolongada. Cada envase contiene un vial de polvo para suspensión inyectable de liberación prolongada, un vial de 3 ml de disolvente, una jeringa con una aguja de seguridad de calibre 19 y de 38 mm adjunta y tres agujas de seguridad separadas; una aguja de calibre 19 y de 38 mm y dos agujas de calibre 19 y de 50 mm.
Titular de la autorización de comercialización
CHEPLAPHARM Registration GmbH, Weilerstr. 5e, 79540 Lörrach, Alemania.
Responsable de la fabricación
Lilly S.A., Avda. de la Industria 30, 28108 Alcobendas, Madrid, España.
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos http://www.ema.europa.eu/.
INSTRUCCIONES PARA LOS PROFESIONALES SANITARIOS
INSTRUCCIONES PARA LA RECONSTITUCIÓN Y ADMINISTRACIÓN
ZYPADHERA olanzapina polvo y disolvente para suspensión inyectable de liberación prolongada
SÓLO PARA INYECCIÓN INTRAMUSCULAR PROFUNDA EN EL GLÚTEO.
NO ADMINISTRAR POR VÍA INTRAVENOSA O SUBCUTÁNEA.
Reconstitución
PASO 1: Preparación de los materiales
El envase incluye:
- Vial de ZYPADHERA polvo para suspensión inyectable de liberación prolongada
- Vial de disolvente para ZYPADHERA
- Una jeringa Hipodérmica y una aguja de seguridad (dispositivo Hipodérmico)
- Una aguja de seguridad Hipodérmica del calibre 19, de 38 mm
- Dos agujas de seguridad Hipodérmicas del calibre 19, de 50 mm
- Prospecto
- Tarjeta de instrucciones para la Reconstitución y Administración (este documento)
- Información de Seguridad e Instrucciones de Uso del Dispositivo Hipodérmico
|
Se recomienda la utilización de guantes ya que ZYPADHERA puede provocar irritación de la piel.
Reconstituir ZYPADHERA polvo para suspensión inyectable de liberación prolongada exclusivamente con el disolvente suministrado en el envase mediante técnicas asépticas estándar para la reconstitución de productos parenterales.
PASO 2: Determinación del volumen de disolvente para la reconstitución
Esta tabla indica la cantidad de disolvente necesaria para reconstituir ZYPADHERA polvo para suspensión inyectable de liberación prolongada.
Concentración del vial de ZYPADHERA (mg) | Volumen de disolvente a añadir (ml) |
210 | 1,3 |
300 | 1,8 |
405 | 2,3 |
Es importante resaltar que el vial contiene más disolvente del necesario para reconstituir el producto.
PASO 3: Reconstitución de ZYPADHERA
- Golpear suavemente el vial para soltar el polvo.
- Abrir la jeringa Hipodérmica y la aguja pre empaquetada con el dispositivo de protección para agujas. Abrir la bolsa de plástico y sacar el dispositivo. Unir la jeringa (si aún no lo está) al conector tipo Luer del dispositivo con un giro sencillo. Colocar la aguja firmemente sobre el dispositivo empujándolo y girándolo en el sentido de las agujas del reloj. A continuación, retire directamente la capucha de la aguja. Si no se siguen estas instrucciones, puede producirse una lesión por pinchazo de aguja.
- Retirar el volumen de disolvente pre determinado (Paso 2) dentro de la jeringa.
- Inyectar el volumen de disolvente requerido en el vial de polvo.
- Retirar el aire para igualar la presión en el vial.
- Retirar la aguja, con el vial hacia arriba para impedir la pérdida de disolvente.
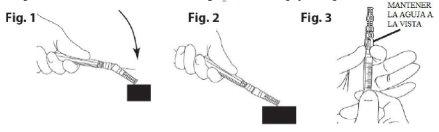
- Poner el dispositivo de seguridad para agujas. Colocar la aguja en su funda utilizando una técnica con una sola mano. Realizar esta maniobra con una mano haciendo una presión SUAVE de la funda contra una superficie plana. AL PRESIONAR SOBRE LA FUNDA (Fig. 1), LA AGUJA SE CONECTA A ELLA FIRMEMENTE (Fig. 2)
- Confirmar visualmente que la aguja está completamente conectada a su funda protectora. Retirar el dispositivo con la aguja sujeta a la jeringa, cuando sea requerido, mediante un procedimiento médico específico. Retirarlo sujetando el conector tipo Luer del dispositivo de protección de la aguja con ayuda de los dedos pulgar e índice, y dejando los otros tres dedos restantes alejados del dispositivo donde se encuentra la punta de la aguja (Fig. 3).
|
- Golpear el vial de forma vigorosa repetidas veces sobre una superficie dura hasta que no quede polvo visible. Proteger la superficie para amortiguar el impacto. (Ver Figura A)
|
Figura A: Golpear vigorosamente para mezclar
- Comprobar el vial visualmente para identificar apelmazamiento del polvo. El polvo que no está en suspensión tiene un aspecto de terrones secos de color amarillo pálido adheridos al vial. Puede ser necesario seguir golpeando si permanecen terrones. (Ver Figura B)
|
Sin suspender: terrones visibles Suspendido: sin terrones
Figura B: Verificar si hay polvo sin suspender y seguir golpeando si es necesario.
- Agitar el vial enérgicamente hasta que la suspensión tenga un aspecto uniforme con color y texturas homogéneos. El producto suspendido aparecerá amarillo y opaco. (Ver Figura C)
|
Figura C: Agitar el vial vigorosamente
Si se forma espuma, deje reposar el vial para que se disipe la espuma. Si no se utiliza el producto de forma inmediata, debe agitarse de forma vigorosa para conseguir la resuspensión. ZYPADHERA reconstituido permanece estable en el vial hasta un máximo de 24 horas.
Administración
PASO 1: Inyectar ZYPADHERA
Esta tabla confirma el volumen final de ZYPADHERA en suspensión que hay que inyectar. La concentración de la suspensión es 150 mg/ml de olanzapina.
Dosis (mg) | Volumen final a inyectar (ml) |
150 | 1,0 |
210 | 1,4 |
300 | 2,0 |
405 | 2,7 |
- Determinar qué aguja se va a emplear para administrar al paciente la inyección. Para los pacientes obesos, se recomiendan para la inyección agujas de 50 mm:
- Si se emplea la aguja de 50 mm para la inyección, ponga la aguja de seguridad de 38 mm a la jeringa para retirar el volumen de suspensión requerido.
- Si se emplea la aguja de 38 mm para la inyección, ponga la aguja de seguridad de 50 mm para retirar el volumen de suspensión requerido.
- Retirar lentamente la cantidad deseada. Quedará un poco de producto sobrante en el vial.
- Poner el dispositivo de seguridad para agujas y retirar la aguja de la jeringa.
- Poner la aguja de seguridad, seleccionando la de 50 mm o la de 38 mm, en la jeringa antes de la inyección. Una vez extraída la suspensión del vial y pasada a la jeringa, debe inyectarse de forma inmediata.
- Seleccionar y preparar el lugar de la inyección en la zona glútea. NO INYECTAR POR VÍA INTRAVENOSA O SUBCUTÁNEA.
- Después de insertar la aguja, aspirar durante unos segundos para confirmar que no sale nada de sangre. Si se aspira sangre dentro de la jeringa, descartar la jeringa y preparar una nueva suspensión. La inyección deberá realizarse con presión firme y continua.
NO MASAJEAR EL LUGAR DE LA INYECCIÓN.
- Poner el dispositivo de seguridad de agujas. (Fig. 1 y 2)
- Descartar los viales, jeringa, agujas usadas, aguja adicional y el disolvente sobrante de acuerdo con los procedimientos clínicos adecuados. El vial es de un solo uso.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ZYPADHERA 300 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADAForma farmacéutica: COMPRIMIDO, 10 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 2,5 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere recetaForma farmacéutica: COMPRIMIDO, 5 mgPrincipio activo: OlanzapinaFabricante: Neuraxpharm Spain S.L.Requiere receta
Médicos online para ZYPADHERA 300 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ZYPADHERA 300 mg POLVO Y DISOLVENTE PARA SUSPENSION INYECTABLE DE LIBERACION PROLONGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes