РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ

Спросите врача о рецепте на РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ

Инструкция по применению РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ
Введение
Инструкция: информация для пользователя
Ривастигмина Аресто 9,5мг/24ч пластырьытрансдермальныеыЕФГ
Прочитайте внимательно всю инструкцию перед началом использования этого лекарства, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться прочитать ее снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или медсестрой.
- Это лекарство было назначено только вам, и не передавайте его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или медсестрой, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции:
- Что такое Ривастигмина Аресто и для чего оно используется2. Что вам нужно знать перед началом использования Ривастигмина Аресто
- Как использовать Ривастигмина Аресто
- Возможные побочные эффекты
- Хранение Ривастигмина Аресто
- Содержание упаковки и дополнительная информация
1. Что такое Ривастигмина Аресто и для чего оно используется
Активное вещество Ривастигмина Аресто - ривастигмина.
Ривастигмина относится к группе ингибиторов холинэстеразы. У пациентов с деменцией Альцгеймера определенные нервные клетки умирают в мозге, что приводит к низким уровням нейротрансмиттеров ацетилхолина (вещества, позволяющей нервным клеткам общаться друг с другом). Ривастигмина действует, блокируя ферменты, разрушающие ацетилхолин: ацетилхолинэстеразу и бутирилхолинэстеразу. Блокируя эти ферменты, ривастигмина позволяет увеличить уровень ацетилхолина в мозге, помогая уменьшить симптомы болезни Альцгеймера.
Ривастигмина Аресто используется для лечения взрослых пациентов с деменцией Альцгеймера легкой или умеренной степени, прогрессивным заболеванием мозга, которое постепенно влияет на память, интеллектуальные способности и поведение.
2. Что вам нужно знать перед началом использования Ривастигмина Аресто
Не используйте Ривастигмина Аресто
- если вы аллергичны к ривастигмине (активному веществу Ривастигмина Аресто) или к любому другому компоненту этого лекарства (перечисленному в разделе 6).
- если вы когда-либо имели аллергическую реакцию на подобное лекарство (производные карбамата).
- если у вас есть кожная реакция, распространяющаяся за пределы размера пластыря, если есть более сильная местная реакция (такая как пузыри, воспаление кожи, отек) и если нет улучшения в течение 48 часов после удаления трансдермального пластыря.
Если вы находитесь в одной из этих ситуаций, сообщите об этом вашему врачу и не используйте Ривастигмина Аресто трансдермальные пластыри.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом перед началом использования Ривастигмина Аресто:
- если у вас есть или были когда-либо проблемы с сердцем, такие как нерегулярный сердечный ритм (пульс) или замедленный, удлинение QTc, семейная история удлинения QTc, torsade de pointes, или если у вас низкий уровень калия или магния в крови.
- если у вас есть или были когда-либо язвы желудка.
- если у вас есть или были когда-либо проблемы с мочеиспусканием.
- если у вас есть или были когда-либо судороги.
- если у вас есть или были когда-либо бронхialная астма или тяжелое респираторное заболевание.
- если у вас есть тремор.
- если у вас низкий вес.
- если у вас есть желудочно-кишечные реакции, такие как тошнота, рвота и диарея. Вы можете обезвожиться (потерять большое количество жидкости), если рвота или диарея продолжаются.
- если у вас есть проблемы с печенью (печеночная недостаточность).
Если вы находитесь в одной из этих ситуаций, ваш врач может рассмотреть необходимость более тщательного наблюдения во время лечения.
Если вы не использовали пластыри более трех дней, не накладывайте новый, не проконсультировавшись с вашим врачом.
Дети и подростки
Ривастигмина Аресто не должно использоваться в педиатрической популяции для лечения болезни Альцгеймера.
Ривастигмина Аресто с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы используете, недавно использовали или можете использовать любое другое лекарство.
Ривастигмина Аресто может взаимодействовать с антихолинергическими лекарствами, некоторые из которых используются для облегчения спазмов или судорог желудка (например, дцикломин), для лечения болезни Паркинсона (например, амантадин) или для предотвращения укачивания (например, дифенгидрамин, скополамин или меклизин).
Ривастигмина Аресто трансдермальные пластыри не должны применяться одновременно с метоклопрамидом (лекарством, используемым для облегчения или предотвращения тошноты и рвоты). Одновременное применение этих двух лекарств может вызвать проблемы, такие как жесткость конечностей и тремор рук.
Если вам необходимо пройти хирургическую операцию во время использования Ривастигмина Аресто трансдермальных пластырей, сообщите вашему врачу, что вы используете это лекарство, поскольку оно может чрезмерно усилить эффекты некоторых миорелаксантов анестезии.
Следует быть осторожным при использовании Ривастигмина Аресто трансдермальных пластырей с бета-блокаторами (лекарствами, такими как атенолол, используемыми для лечения гипертонии, стенокардии и других сердечных заболеваний). Одновременное применение этих двух лекарств может вызвать осложнения, такие как замедление сердечного ритма (брадикардия), которое может привести к обмороку или потере сознания.
Следует быть осторожным при использовании Ривастигмина Аресто с другими лекарствами, которые могут влиять на сердечный ритм или электрическую систему сердца (удлинение QT).
Беременность, лактация и фертильность
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Если вы беременны, необходимо оценить преимущества использования Ривастигмина Аресто по сравнению с возможными побочными эффектами для плода. Ривастигмина Аресто не должно использоваться во время беременности, если это не абсолютно необходимо.
Не кормите грудью во время лечения Ривастигмина Аресто трансдермальными пластырями.
Вождение и использование машин
Ваш врач сообщит вам, позволяет ли ваше заболевание вам безопасно управлять транспортными средствами или использовать машины. Ривастигмина Аресто трансдермальные пластыри могут вызывать головокружение и сильную дезориентацию. Если вы чувствуете головокружение или дезориентацию, не управляйте транспортными средствами и не используйте машины или не выполняйте другие задачи, требующие вашего внимания.
3. Как использовать Ривастигмина Аресто
Следуйте точно инструкциям по применению Ривастигмина Аресто трансдермальных пластырей, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
ВАЖНО:
- Удалите предыдущий пластырь перед применением НОВОГО пластыря.
- Применяйте только ОДИН пластырь в день.
- Не разрезайте пластырь на части.
- Прижимайте пластырь плотно к коже ладонью руки не менее 30 секунд.
Как начать лечение
Ваш врач укажет вам наиболее подходящую дозу Ривастигмина Аресто трансдермального пластыря.
- Обычно лечение начинается с Ривастигмина Аресто 4,6 мг/24 ч.
- Рекомендуемая суточная доза составляет Ривастигмина Аресто 9,5 мг/24 ч. Если эта доза хорошо переносится, ваш врач может рассмотреть возможность увеличения дозы до 13,3 мг/24 ч. (Доза 13,3 мг/24 ч не может быть достигнута с помощью этого продукта. Для условий, при которых должна использоваться эта доза, проконсультируйтесь с другими продуктами ривастигмины, для которых доступны трансдермальные пластыри 13,3 мг/24 ч).
- Применяйте только один трансдермальный пластырь одновременно и замените пластырь на новый через 24 часа.
Во время лечения ваш врач может корректировать дозу в зависимости от ваших индивидуальных потребностей.
Если вы не использовали пластыри более трех дней, не накладывайте новый, не проконсультировавшись с вашим врачом. Лечение трансдермальным пластырем можно возобновить с той же дозы, если лечение не было прервано более чем на три дня. В противном случае ваш врач может назначить вам начать лечение с Ривастигмина Аресто 4,6 мг/24 ч.
Ривастигмина Аресто трансдермальный пластырь можно использовать с пищей, напитками и алкоголем.Где применять Ривастигмина Аресто пластырь
- Перед применением пластыря убедитесь, что кожа чистая, сухая и без волос, без порошков, масла, увлажнителя или лосьона, которые могут препятствовать адгезии пластыря к коже, без порезов, покраснений или раздражений.
- Аккуратно удалите любой предыдущий пластырь перед применением нового.Ношение нескольких пластырей на вашем теле может привести к чрезмерному воздействию этого лекарства и быть потенциально опасным.
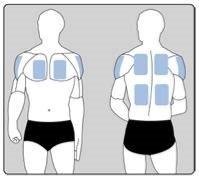
- Применяйте только ОДИН пластырь в день на ОДНОЙ из возможных зон, как показано на следующих схемах:
- верхняя левая или верхняя правая часть руки
- верхняя левая или верхняя правая часть груди (избегая молочных желез у женщин)
- верхняя левая или верхняя правая часть спины
- нижняя левая или нижняя правая часть спины
|
Каждые 24 часа удалите предыдущий пластырь перед применением НОВОГО пластыря на ОДНОЙ из возможных зон.
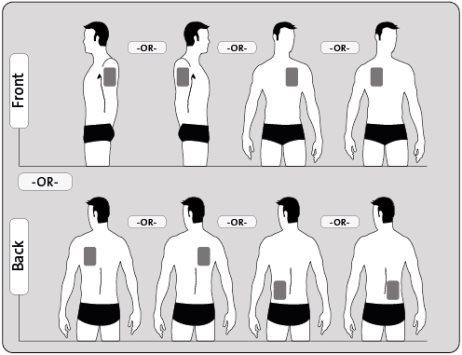
Каждый раз, когда вы меняете пластырь, удалите предыдущий пластырь с кожи перед применением нового пластыря на другой участок кожи (например, один день на правой стороне тела, а на следующий день - на левой стороне; или один день на верхней части тела, а на следующий день - на нижней части). Подождите не менее 14 дней, прежде чем применить новый пластырь на том же участке кожи.
Как применять Ривастигмина Аресто пластырь
Пластыри Ривастигмина Аресто тонкие, коричневые и прикрепляются к коже. Каждый пластырь находится в отдельном пакете, который защищает его до момента применения. Не открывайте пакет и не удаляйте пластырь, пока не будете готовы его применить.
| Аккуратно удалите существующий пластырь перед применением нового. Пациенты, начинающие лечение впервые, и пациенты, возобновляющие лечение ривастигминой после перерыва, должны начать с второй схемы. |
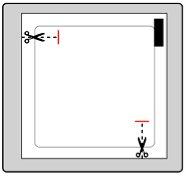
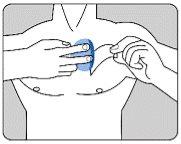
| Каждый пластырь находится в отдельном защитном пакете. Открывайте пакет только перед применением пластыря. Разрежьте пакет по обеим меткам ножниц. Разорвите пакет, чтобы открыть его. Не разрезайте весь пакет, чтобы не повредить пластырь. Удалите пластырь из пакета. |
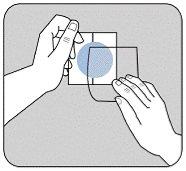
| Удалите кожноцветную защитную пленку с верхней части пластыря и выбросьте ее. Двухчастная защитная пленка покрывает адгезивную сторону пластыря. Удалите первую часть защитной пленки, не прикасаясь пальцами к адгезивной стороне пластыря. |
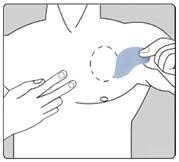
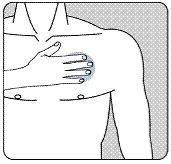
| Поместите адгезивную сторону пластыря на верхнюю или нижнюю часть спины, на верхнюю часть руки или на грудь, а затем удалите вторую часть защитной пленки. |
| Прижимайте пластырь плотно к коже ладонью руки не менее 30 секунд и убедитесь, что края хорошо приклеены. |
Если это помогает, вы можете написать на пластыре, например, день недели, с помощью тонкого маркера.
Вы должны носить пластырь постоянно до момента его замены на новый. Когда вы будете применять новый пластырь, вы можете попробовать разные зоны, чтобы найти те, которые будут для вас наиболее удобными и где одежда не будет тревожить пластырь.
Как удалить Ривастигмина Аресто пластырь
Аккуратно потяните за один из краев пластыря, чтобы медленно удалить его с кожи. Если остаются остатки адгезива на коже, смочите область теплой водой и мягким мылом или используйте детское масло, чтобы удалить его. Не используйте алкоголь или другие растворители (например, жидкости для снятия лака или другие растворители).
После удаления пластыря руки должны быть вымыты с мылом или водой. В случае контакта с глазами или если глаза покраснеют после обращения с пластырем, промойте их большим количеством воды и проконсультируйтесь с врачом, если симптомы не исчезнут.
Можно ли носить Ривастигмина Аресто пластырь во время купания, плавания или воздействия солнца?
- Купание, плавание или душ не должны повлиять на пластырь. Убедитесь, что он не отсоединяется частично во время этих занятий.
- Не подвергайте пластырь воздействию внешних источников тепла (например, чрезмерного солнечного света, сауны, солярия) в течение длительного времени.
Что делать, если пластырь отсоединился
Если пластырь отсоединился, примените новый на остаток дня и замените его на следующий день в обычное время.
Когда и как долго следует носить Ривастигмина Аресто пластырь?
- Чтобы получить пользу от лечения, примените новый пластырь каждый день, желательно в одно и то же время.
- Носите только один пластырь Ривастигмина Аресто одновременно и замените пластырь на новый через 24 часа.
Если вы применили больше Ривастигмина Аресто, чем следует
Если вы случайно применили более одного пластыря, удалите все пластыри с кожи и сообщите об этом вашему врачу или позвоните в Центр токсикологической информации, телефон: 91 562 04 20 (указав лекарство и количество, принятое перорально). Вам может потребоваться медицинская помощь. Некоторые люди, которые случайно приняли слишком большое количество ривастигмины перорально, испытывали тошноту, рвоту, диарею, повышение артериального давления и галлюцинации. Также могут возникнуть замедление сердечного ритма и обморок.
Если вы забыли использовать Ривастигмина Аресто
Если вы обнаружите, что забыли применить пластырь, примените его сразу же. На следующий день примените следующий пластырь в обычное время. Не применяйте два пластыря, чтобы компенсировать пропущенный.
Если вы прервете лечение Ривастигмина Аресто
Сообщите вашему врачу или фармацевту, если вы прекратите использовать пластыри.
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, Ривастигмина Аресто трансдермальные пластыри могут вызывать побочные эффекты, хотя не все люди испытывают их.
У вас могут быть побочные эффекты чаще при начале лечения или когда увеличивается доза. Обычно побочные эффекты медленно исчезают, когда ваш организм привыкает к лекарству.
Если вы заметите любой из следующих побочных эффектов, которые могут быть серьезными, снимите пластырь и немедленно сообщите врачу:
Частые(могут возникать у до 1 из 10 человек)
- Потеря аппетита
- Чувство головокружения
- Чувство беспокойства или онемения
- Неспособность контролировать мочеиспускание (недержание мочи)
Редкие(могут возникать у до 1 из 100 человек)
- Проблемы с сердечным ритмом, такие как замедленный сердечный ритм
- Видение вещей, которые на самом деле не существуют (галлюцинации)
- Язва желудка
- Обезвоживание (потеря большого количества жидкости)
- Гиперактивность (высокий уровень активности, беспокойство)
- Агрессивность
Очень редкие(могут возникать у до 1 из 1000 человек)
- Падения
Чрезвычайно редкие(могут возникать у до 1 из 10 000 человек)
- Жесткость рук и ног
- Дрожание в руках
Неизвестная частота(не может быть оценена из доступных данных)
- Аллергическая реакция в месте применения пластыря, такая как пузыри или воспаление кожи
- Ухудшение симптомов болезни Паркинсона - таких как дрожание, жесткость и трудности с движением
- Синдром Пиза (состояние, характеризующееся непроизвольным сокращением мышц и аномальным наклоном тела и головы в сторону)
- Воспаление поджелудочной железы - симптомы включают боль в верхней части живота, часто сопровождаемую чувством тошноты (рвотой) или головокружением
- Быстрый или нерегулярный сердечный ритм
- Высокое кровяное давление
- Эпилептические приступы (судороги)
- Повреждения печени (желтуха кожи, желтуха белого глаза, необычное потемнение мочи или необъяснимая тошнота, рвота, усталость и потеря аппетита)
- Изменения в анализах, показывающих функцию печени
- Чувство беспокойства
- Кошмары
Если вы заметите любой из побочных эффектов, перечисленных выше, снимите пластырь и немедленно сообщите врачу.
Другие побочные эффекты, испытанные при приеме капсул или раствора для приема внутрь ривастигмина, и которые могут возникнуть при использовании пластырей:
Частые(могут возникать у до 1 из 10 человек)
- Избыточная слюна
- Потеря аппетита
- Чувство беспокойства
- Чувство общего дискомфорта
- Дрожание или чувство путаницы
- Увеличение потоотделения
Редкие(могут возникать у до 1 из 100 человек)
- Нерегулярный сердечный ритм (например, быстрый сердечный ритм)
- Трудности с засыпанием
- Случайные падения
Очень редкие(могут возникать у до 1 из 1000 человек)
- Эпилептические приступы (судороги)
- Язва в кишечнике
- Боль в груди - вероятно, вызванная спазмом сердца
Чрезвычайно редкие(могут возникать у до 1 из 10 000 человек)
- Высокое кровяное давление
- Воспаление поджелудочной железы - симптомы включают сильную боль в верхней части живота, часто сопровождаемую чувством тошноты (рвотой) или головокружением
- Кровотечение в желудочно-кишечном тракте - проявляется как кровь в кале или при рвоте
- Видение вещей, которые на самом деле не существуют (галлюцинации)
- Некоторые люди, которые испытывали сильную тошноту (рвоту), имели разрыв части пищеварительного тракта, соединяющего рот с желудком (пищевод)
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с врачом, даже если это возможные побочные эффекты, которые не перечислены в этом описании. Вы также можете сообщить о них напрямую через Систему испанского фармаконадзора для лекарств для человека, веб-сайт: www.notificaRAM.es. Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение Ривастигмина Аресто
- Храните это лекарство в недоступном для детей месте.
- Не используйте это лекарство после истечения срока годности, указанного на коробке и упаковке после CAD/EXP. Срок годности - последний день месяца, указанного.
- Это лекарство не требует специальных условий хранения.
- Храните трансдермальный пластырь в упаковке до использования.
- Не используйте никакой пластырь, если вы заметите, что он поврежден или имеет признаки вскрытия.
- После снятия пластыря сложите его пополам с липкой стороной внутрь и прижмите. После помещения в оригинальную упаковку, избавляясь от пластыря, убедитесь, что он находится вне досягаемости детей. После снятия пластыря не трогайте глаза и тщательно мойте руки с мылом и водой. Если ваш мусор утилизируется путем сжигания, вы можете выбросить пластырь в домашний мусор. Если нет, принесите использованные пластыри в аптеку, желательно в оригинальной упаковке.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт сбора SIGRE в аптеке. В случае сомнений спросите у вашего фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Ривастигмина Аресто
- Активное вещество - ривастигмина.
Ривастигмина Аресто 9,5 мг/24 часа трансдермальный пластырь:
Каждый трансдермальный пластырь выпускает 9,5 мг ривастигмины в течение 24 часов, имеет размер 9,2 см2 и содержит 13,8 мг ривастигмины.
- Другие компоненты:
Матрица:
- поли[(2-этилгексил)акрилат, винилацетат]
- полиизобутен среднего и высокого молекулярного веса
- коллоидный алюмосиликат
- жидкий вазелиновый масло
Поддерживающая пленка:
- пленка из полиэтилена/термопластичной смолы/полиэстера, покрытая алюминием
Пленка, выпускающая активное вещество:
- пленка из полиэстера, покрытая фторполимером
Оранжевая печатная краска
Внешний вид продукта и содержание упаковки
Каждый трансдермальный пластырь - тонкий пластырь. Внешний слой имеет коричневый цвет с оранжевым напечатанным текстом:
- "РИВ-ТДС 9,5 мг/24 часа"
Каждая упаковка содержит один трансдермальный пластырь. Каждый трансдермальный пластырь защищен оберткой. Пластыри доступны в упаковках, содержащих 7, 30 или 42 упаковки, и в мультиупаковках, содержащих 60, 84 или 90 упаковок. Возможно, не все размеры упаковок будут доступны.
Владелец разрешения на маркетинг
Аристо Фарма Иберия, С.Л.
улица Солана, 26
28850 - Торрехон де Ардос
Испания
Производитель
Луе Фарма АГ
Ам Виндфельд, 35
83714 Мизбах, Германия
Блюфарма Индустрия Фармацевтика, С.А.
Сан-Мартинью-ду-Бишпу
3045-016 Коимбра
Португалия
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Германия Демелора 9,5 мг/24 часа трансдермальный пластырь
Испания Ривастигмина Аресто 9,5 мг/24 часа трансдермальные пластыри
Франция Ривастигмин Арроу 9,5 мг/24 часа трансдермальное устройство
Италия Демелора
Нидерланды Ривастигмина Ауробиндо 9,5 мг/24 часа пластырь для трансдермального использования
Португалия Ривастигмина Блюфарма
Дата последнего пересмотра этого описания:февраль 2025 г.
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям Испании (AEMPS) http://www.aemps.gob.es/
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 13,3 мг/24 чАктивное вещество: РивастигминПроизводитель: Esteve Pharmaceuticals S.A.Требуется рецептФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 4,6 мг/24 чАктивное вещество: РивастигминПроизводитель: Esteve Pharmaceuticals S.A.Требуется рецептФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 9,5 мг/24 чАктивное вещество: РивастигминПроизводитель: Esteve Pharmaceuticals S.A.Требуется рецепт
Аналоги РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в Польша
Аналог РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в Украина
Врачи онлайн по РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на РИВАСТИГМИНА АРИСТО 9,5 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ – по решению врача и с учетом местных правил.