
НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ
Спросите врача о рецепте на НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ

Инструкция по применению НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ
Введение
Инструкция:информация для пользователя
Небиволол ратиофарм 5 мг таблетки ЕФГ
Прочитайте внимательно всю инструкцию перед началом приема этого лекарства,поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться перечитать ее.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и не передавайте его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции:
- Что такое Небиволол ратиофарм и для чего он используется
- Что вам нужно знать перед началом приема Небиволола ратиофарма
- Как принимать Небиволол ратиофарм
- Возможные побочные эффекты
- Хранение Небиволола ратиофарма
- Содержание упаковки и дополнительная информация
1. Что такое Небиволол ратиофарм и для чего он используется
Небиволол ратиофарм содержит небиволол, лекарство с действием на сердечно-сосудистую систему, принадлежащее к группе селективных бета-блокирующих агентов (с избирательным действием на сердечно-сосудистую систему). Он предотвращает увеличение частоты сердечных сокращений и контролирует силу сердечных сокращений. Также он оказывает расширяющее действие на кровеносные сосуды, что в свою очередь способствует снижению артериального давления.
Он используется для лечения повышенного артериального давления (гипертонии).
Небиволол также используется для лечения легкой и умеренной хронической сердечной недостаточности у пациентов в возрасте 70 лет и старше, в комбинации с другими лекарствами.
2. Что вам нужно знать перед началом приема Небиволола ратиофарма
Не принимайте Небиволол ратиофарм
- если вы аллергичны к небивололу или к любому из других компонентов этого лекарства (указанных в разделе 6).
- если у вас есть одно или несколько из следующих нарушений:
- пониженное артериальное давление
- тяжелые нарушения кровообращения в руках или ногах
- очень медленный сердечный ритм (менее 60 ударов в минуту)
- другие тяжелые нарушения сердечного ритма (например, атриовентрикулярный блок II и III степени или другие нарушения сердечной проводимости)
- вы недавно пережили эпизод сердечной недостаточности или ухудшение ее, или получаете внутривенную терапию для помощи сердцу после коллапса кровообращения из-за острой сердечной недостаточности
- астма или затрудненное дыхание (в настоящее время или в прошлом)
- феохромоцитома, опухоль, расположенная в верхней части почек (надпочечные железы), которая не лечится
- нарушения функции печени.
- метаболические нарушения (метаболический ацидоз), например, диабетическая кетоацидоз.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом приема небиволола.
Сообщите вашему врачу, если у вас есть какие-либо из следующих проблем:
- анормально медленный сердечный ритм,
- тип боли в груди, вызванный спонтанным спазмом коронарных артерий, называемый принцметаловой ангиной,
- хроническая сердечная недостаточность без лечения,
- атриовентрикулярный блок I степени (легкое нарушение сердечной проводимости, влияющее на сердечный ритм),
- плохая циркуляция крови в руках или ногах, например, болезнь или синдром Рейно,
- хронические респираторные проблемы,
- диабет: это лекарство не влияет на уровень сахара в крови, но может маскировать признаки предупреждения, вызванные снижением уровня сахара в крови (например, сердцебиение, быстрое сердцебиение) и может увеличить риск тяжелой гипогликемии при использовании с некоторыми типами антидиабетических препаратов, называемых сульфонилмочевинами (например, гликуидоном, гликлазидом, глибенкламидом, глипизидом, глимепиридом или толбутамидом).
- гиперактивность щитовидной железы: это лекарство может маскировать признаки, связанные с этим нарушением, такие как аномально высокая частота сердечных сокращений,
- аллергии: это лекарство может усилить вашу реакцию на пыльцу или другие вещества, на которые вы аллергичны,
- если у вас есть или было псориаз (заболевание кожи, характеризующееся розовыми чешуйчатыми пятнами),
- если вам предстоит хирургическое вмешательство, всегда сообщайте вашему анестезиологу, что вы принимаете Небиволол ратиофарм.
Если у вас есть тяжелые нарушения функции почек, проконсультируйтесь с вашим врачом перед началом приема небиволола для лечения сердечной недостаточности.
При начале лечения хронической сердечной недостаточности вам необходимо будет регулярно наблюдаться у врача (см. раздел 3).
Это лечение не должно быть внезапно прекращено, если только это明но не указано и оценено вашим врачом (см. раздел 3).
Дети и подростки
Нерекомендуется использование Небиволола ратиофарма у детей и подростков из-за отсутствия данных об использовании этого лекарства в этой группе пациентов.
Прием Небиволола ратиофармасдругими лекарствами
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принимать любое другое лекарство.
Важно, чтобы вы всегда сообщали вашему врачу, если, кроме небиволола, вы принимаете любое из следующих лекарств:
- Некоторые лекарства для сердца или для контроля артериального давления (например, амиодарон, амлодипин, цибензолин, клонидин, дигоксин, дилтиазем, дисопирамид, фелодипин, флекаинид, гуанфацин, гидрохинидин, лацидипин, лидокаин, метилдопа, мексилетин, мононидин, никардипин, нифедипин, нимодипин, нитрендипин, пропафенон, хинидин, рилменидин, верапамил).
- Седативные и психотические лекарства (психические заболевания), такие как барбитураты (также используемые для эпилепсии), фенотиязин (также используемый для рвоты и тошноты) и тioridazin.
- Лекарства для депрессии, такие как амитриптилин, пароксетин и флуоксетин.
- Лекарства, используемые для анестезии во время операции.
- Лекарства для астмы, деконгестанты или некоторые лекарства для лечения глазных нарушений, таких как глаукома (повышение давления в глазу) или расширение зрачка.
- Баклофен (антиспазматическое лекарство); Амифостин (защитное лекарство, используемое во время лечения рака).
- Лекарства для диабета, такие как инсулин или пероральные антидиабетические препараты
Все эти лекарства, как и небиволол, могут влиять на артериальное давление и функцию сердца.
Лекарства для лечения избыточной кислотности в желудке или язвы (антиацидные лекарства): вы должны принимать небиволол во время еды, а антиацид - между приемами пищи.
ПриемНебиволола ратиофармаспродуктами питанияи напитками
См. раздел 3.
Беременность илактация
Небиволол ратиофарм не должен назначаться во время беременности, если только ваш врач не считает это необходимым.
Не рекомендуется его использование во время лактации.
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете беременность, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Вождение и использование машин
Это лекарство может вызывать головокружение или усталость. Если это происходит, не驾驶айте транспортные средства и не используйте машины.
Небиволол ратиофармсодержит лактозу
Это лекарство содержит лактозу. Если ваш врач указал, что у вас есть непереносимость некоторых сахаров, проконсультируйтесь с ним перед приемом этого лекарства.
Это лекарство содержит менее 1 ммоль натрия (23 мг) на таблетку; это означает, что оно практически не содержит натрия.
3. Как принимать Небиволол ратиофарм
Следуйте точно инструкциям по приему этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Небиволол можно принимать до, во время или после еды, но также его можно принимать независимо от еды. Предпочтительно принимать таблетку с небольшим количеством воды.
Лечение повышенного артериального давления (гипертонии)
- Обычная доза - 1 таблетка в день. Предпочтительно принимать дозу всегда в одно и то же время суток.
- У пациентов пожилого возраста и пациентов с нарушениями функции почек рекомендуется начинать лечение с ½ (половины) таблетки в день.
- Терапевтический эффект на артериальное давление достигается через 1-2 недели лечения. Иногда оптимальный эффект достигается только через 4 недели.
Лечение хронической сердечной недостаточности
- Ваше лечение будет начато и контролироваться врачом с опытом.
- Ваш врач начнет ваше лечение с ¼ (четверти) таблетки в день. Доза будет увеличена после 1-2 недель до ½ (половины) таблетки в день, затем до 1 таблетки в день и, наконец, до 2 таблеток в день до достижения оптимальной дозы для вас. Ваш врач назначит вам правильную дозу на каждом этапе, и вы должны следовать точно его инструкциям.
- Максимальная рекомендуемая доза - 2 таблетки (10 мг) в день.
- Начало лечения и каждое увеличение дозы будут проводиться под наблюдением опытного врача в течение 2 часов.
- Ваш врач уменьшит вашу дозу, если это необходимо.
- Не следует внезапно прекращать лечение, поскольку это может ухудшить вашу сердечную недостаточность.
- Пациенты с тяжелыми нарушениями функции почек не должны принимать это лекарство.
- Принимайте лекарство один раз в день, предпочтительно в одно и то же время суток.
Если ваш врач указал, что вам необходимо принимать ¼ (четверть) или ½ (половину) таблетки в день, следуйте следующим инструкциям для деления таблеток Небиволола ратиофарма, имеющих форму креста:
- Положите таблетки на плоскую, твердую поверхность (например, стол или прилавок), с крестовой формой вверх.
- Разломайте таблетку, нажимая на нее пальцами обеих рук, расположенными по обе стороны от одной из крестовых форм (Рисунки 1 и 2).
- Повторите то же самое, чтобы разделить половину таблетки на четверти (Рисунки 3 и 4).
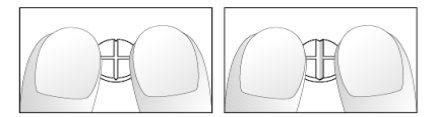
Рисунки 1 и 2: Легкое разломание таблетки Небиволола 5 мг с крестовой формой на половину.

Рисунки 3 и 4: Легкое разломание половины таблетки Небиволола 5 мг с крестовой формой на четверти.
- Ваш врач решит, следует ли комбинировать небиволол с другими лекарствами для лечения вашего заболевания.
- Не давайте детям или подросткам.
Если вы приняли больше Небиволола ратиофарма, чем следует
Если вы случайно приняли слишком большую дозу этого лекарства, немедленно проконсультируйтесь с вашим врачом, фармацевтом или позвоните в Центр токсикологической информации, телефон 91 562 04 20, указав лекарство и количество, принятое. Симптомы и признаки передозировки небиволола включают очень медленный сердечный ритм (брадикардию), низкое артериальное давление с возможностью обморока (гипотензию), затрудненное дыхание, как при астме (бронхоспазм), и острую сердечную недостаточность.
Вы можете принять активированный уголь (который доступен в вашей аптеке), пока ждете прибытия врача.
Если вы забыли принять Небиволол ратиофарм
Если вы забыли принять дозу небиволола, но вспомнили об этом вскоре после того, как должны были принять ее, принимайте дозу как обычно. Однако, если прошло много времени (несколько часов), так что приближается время следующей дозы, пропустите пропущенную дозу и принимайте следующую запланированную дозу в обычное время. Не принимайте двойную дозу. Однако следует избегать повторного забывания приема лекарства.
Если вы прекратите лечение Небивололом ратиофармом
Проконсультируйтесь с вашим врачом перед прекращением лечения Небивололом ратиофармом, будь то для повышенного артериального давления или хронической сердечной недостаточности.
Не следует внезапно прекращать лечение, поскольку это может временно ухудшить вашу сердечную недостаточность. Если необходимо прекратить лечение хронической сердечной недостаточности, суточная доза должна быть постепенно уменьшена, начиная с уменьшения дозы вдвое с интервалом в одну неделю.
Если у вас есть какие-либо другие вопросы об использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Когда небиволол используется для лечения повышенного артериального давления, возможные побочные эффекты включают:
Частые побочные эффекты (может повлиять на до 1 из 10 человек)
- головная боль,
- головокружение,
- усталость,
- необычный зуд или ощущение онемения,
- диарея,
- запор,
- тошнота,
- затрудненное дыхание,
- отек рук или ног
Редкие побочные эффекты (может повлиять на до 1 из 100 человек)
- медленный сердечный ритм или другие нарушения сердечного ритма,
- низкое артериальное давление,
- боль при ходьбе, подобная судороге,
- анормальное зрение,
- импотенция,
- чувство депрессии,
- затрудненное пищеварение (диспепсия), газы в желудке или кишечнике, рвота,
- высыпания на коже, зуд,
- затрудненное дыхание, как при астме, из-за внезапного сужения мышц вокруг дыхательных путей (бронхоспазм),
- кошмары.
Очень редкие побочные эффекты (может повлиять на до 1 из 10 000 человек)
- обморок,
- ухудшение псориаза (заболевание кожи, характеризующееся розовыми чешуйчатыми пятнами).
Следующие побочные эффекты были зарегистрированы только в отдельных случаях во время лечения Небивололом ратиофармом:
- аллергические реакции, такие как обширные кожные высыпания (реакции гиперчувствительности),
- внезапное отекание, особенно в области вокруг губ, глаз и/или языка, которое может сопровождаться затруднением дыхания (ангиоэдем),
- кожные высыпания, характеризующиеся розовыми, приподнятыми пятнами, которые вызывают зуд, аллергического или неаллергического происхождения (уртикария).
В клиническом исследовании по хронической сердечной недостаточности были обнаружены следующие побочные эффекты:
Очень частые побочные эффекты (может повлиять на более 1 из 10 человек)
- медленный сердечный ритм,
- головокружение.
Частые побочные эффекты (может повлиять на до 1 из 10 человек)
- ухудшение сердечной недостаточности,
- низкое артериальное давление (как чувство обморока при быстром подъеме),
- непереносимость этого лекарства,
- легкое нарушение сердечной проводимости, влияющее на сердечный ритм (атриовентрикулярный блок I степени).
- отек нижних конечностей (увеличение объема лодыжек).
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Также вы можете сообщить об этом напрямую через Испанскую систему фармакологической безопасности лекарств для человека: www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Небиволола ратиофарма
Храните это лекарство вне поля зрения и досягаемости детей.
Не используйте это лекарство после даты истечения срока годности, указанной на коробке и блистере после CAD. Дата истечения срока годности - последний день месяца, указанного.
Это лекарство не требует специальных условий хранения.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт SIGRE вашей аптеки. Если у вас есть сомнения, проконсультируйтесь с вашим фармацевтом, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
СоставНебиволола ратиофарма
- Активное вещество - небиволол.
Каждая таблетка содержит 5 мг небиволола, что эквивалентно 5,45 мг небиволола гидрохлорида.
- Другие компоненты - коллоидный анидрид кремнезема, стеарат магния, кроскармеллоза натрия, макрогол 6000 и лактоза моногидрат.
Внешний вид продукта и содержание упаковки
Таблетки круглые, белые, выпуклые, диаметром 9 мм с крестовой формой на одной стороне и маркировкой "N 5" на другой стороне.
Таблетки можно делить на половину и четверть.
Упаковка:
Блистер: 10, 14, 28, 30 и 90 таблеток.
Флакон из HDPE: 10, 14, 28, 30 и 90 таблеток.
Возможно, не все размеры упаковки будут продаваться.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг
Teva Pharma, S.L.U.
К/ Анабель Сегура, 11. Здание Альбатрос Б, 1-й этаж
28108 Алькобендас, Мадрид
Испания
Производитель
Balkanpharma Dupnitsa AD
3 Самоковско Шоссе Ст.
Дупница 2600
Болгария
или
Actavis Ltd.
BLB015-016 Bulebel Industrial Estate
Зейтун ZTN 3000
Мальта
Дата последней ревизии этойинструкции:Май 2025
Подробная информация о этом лекарстве доступна на сайте Испанского агентства по лекарствам и медицинским изделиям (AEMPS) (http://www.aemps.gob.es/)

Сколько стоит НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ в Испании в 2026 году?
Средняя цена на НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ в январь, 2026 года составляет около 7.87 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках7.87 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИФорма выпуска: ТАБЛЕТКА, 10 мгАктивное вещество: небивололПроизводитель: Glenmark Arzneimittel GmbhТребуется рецептФорма выпуска: ТАБЛЕТКА, 2.5 мгАктивное вещество: небивололПроизводитель: Glenmark Arzneimittel GmbhТребуется рецептФорма выпуска: ТАБЛЕТКА, 5 мгАктивное вещество: небивололПроизводитель: Glenmark Arzneimittel GmbhТребуется рецепт
Аналоги НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ в Польша
Аналог НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ в Украина
Врачи онлайн по НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на НЕБИВОЛОЛ РАТИОФАРМ 5 мг ТАБЛЕТКИ – по решению врача и с учетом местных правил.










