
NEBIVOLOL AUROVITAS 5 mg TABLETS

How to use NEBIVOLOL AUROVITAS 5 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Nebivolol Aurovitas 5 mg Tablets EFG
Readthe entire package leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Nebivolol Aurovitas and what is it used for
- What you need to know before taking Nebivolol Aurovitas
- How to take Nebivolol Aurovitas
- Possible side effects
- Storage of Nebivolol Aurovitas
- Contents of the pack and other information
1. What is Nebivolol Aurovitas and what is it used for
Nebivolol Aurovitas tablets contain nebivolol, a medicine with cardiovascular action belonging to the group of selective beta-blockers (with selective activity in the cardiovascular system). It prevents an increase in heart rate and controls the heart's pumping force. It also has a vasodilating effect, which in turn helps to lower blood pressure.
It is used to treat high blood pressure (hypertension).
Nebivolol Aurovitas is also used to treat mild and moderate chronic heart failure in patients aged 70 or older, in combination with other medicines.
2. What you need to know before taking Nebivolol Aurovitas
Do not take Nebivolol Aurovitas
- If you are allergic to nebivolol or any of the other ingredients of this medicine (listed in section 6).
- If you have any of the following conditions:
- Low blood pressure.
- Severe circulation problems in your arms or legs.
- A very slow heart rate (less than 60 beats per minute).
- Other severe heart rhythm disorders (e.g., second and third degree atrioventricular block, cardiac conduction disorders).
- Heart failure that has recently worsened or if you are receiving intravenous treatment to help your heart work after suffering a circulatory collapse due to acute heart failure.
- Asthma or difficulty breathing (currently or in the past).
- Pheochromocytoma, a tumor located in the upper part of the kidneys (adrenal glands), that is not being treated.
- Liver function disorder.
- Metabolic disorder (metabolic acidosis), such as diabetic ketoacidosis.
Warnings and precautions
Talk to your doctor or pharmacist before starting to take Nebivolol Aurovitas.
Tell your doctor if you have or develop any of the following problems:
- An abnormally slow heart rate.
- A type of chest pain due to a spontaneous spasm of the heart's arteries, called Prinzmetal's angina.
- Untreated chronic heart failure.
- First-degree heart block (a type of mild cardiac conduction disorder that affects the heart rhythm).
- Poor circulation in your arms or legs, e.g., Raynaud's disease or syndrome, pain when walking similar to a cramp.
- Chronic respiratory problems.
- Diabetes: this medicine has no effect on blood sugar levels, but it can mask the warning signs of low blood sugar levels (e.g., palpitations, rapid heart rate).
- Overactive thyroid gland: this medicine can mask the symptoms of rapid heart rate due to this condition.
- Allergy: this medicine can increase your reaction to pollen or other substances you are allergic to.
- If you have or have had psoriasis (a skin disease characterized by scaly pink patches).
- If you need to undergo surgery, always inform your anesthesiologist that you are taking nebivolol before being anesthetized.
If you have severe kidney problems, do not take nebivolol to treat heart failure, and consult your doctor.
When starting treatment for chronic heart failure, you will need to be regularly monitored by a doctor (see section 3).
This treatment should not be stopped abruptly unless clearly indicated and evaluated by your doctor (see section 3).
Children and adolescents
Do notrecommend the use of Nebivolol Aurovitas in children and adolescents due to the lack of data on the use of this medicine in these patients.
Other medicines and Nebivolol Aurovitas
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Always tell your doctor or pharmacist if, in addition to Nebivolol Aurovitas, you are taking any of the following medicines:
- Medicines to control blood pressure or medicines used for heart problems (such as amiodarone, amlodipine, cibenzoline, clonidine, digoxin, diltiazem, disopyramide, felodipine, flecainide, guanfacine, hydroquinidine, lacidipine, lidocaine, methyldopa, mexiletine, moxonidine, nifedipine, nicardipine, nimodipine, nitrendipine, propafenone, quinidine, rilmenidine, verapamil).
- Sedatives and medicines used to treat psychosis (mental illness), such as barbiturates (also used to treat epilepsy), phenothiazine (also used for vomiting and nausea), and thioridazine.
- Medicines to treat depression, such as amitriptyline, paroxetine, and fluoxetine.
- Medicines used for anesthesia during an operation.
- Medicines for asthma, decongestants, or certain medicines for eye disorders such as glaucoma (increased pressure in the eye) or pupil dilation.
- Baclofen (a muscle relaxant); amifostine (a protective medicine used during cancer treatment).
All these medicines, like nebivolol, can affect blood pressure and/or heart function.
- Medicines to treat excess stomach acid or ulcers (antacids), you should take this medicine during meals and the antacid between meals.
Taking Nebivolol Aurovitas with food and drinks
See section 3.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
Nebivolol should not be taken during pregnancy unless clearly necessary.
Breastfeeding
Breastfeeding is not recommended during treatment with nebivolol.
Driving and using machines
This medicine can cause dizziness or fatigue. If this happens, do not drive or use machines.
Nebivolol Aurovitas contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to some sugars, consult them before taking this medicine.
Nebivolol Aurovitas contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per tablet; this is essentially "sodium-free".
3. How to take Nebivolol Aurovitas
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Nebivolol Aurovitas can be taken before, during, or after meals. But it can also be taken independently of them. It is preferable to take the tablet with a little water.
Treatment of high blood pressure (hypertension):
- The recommended dose is 5 mg per day (one 5 mg tablet or two 2.5 mg tablets). It is preferable to take the dose always at the same time of day.
- Elderly patients and those with kidney problems will normally start treatment with 2.5 mg per day (half a 5 mg tablet or one 2.5 mg tablet).
- The therapeutic effect on blood pressure is achieved after 1-2 weeks of treatment. Occasionally, the optimal effect is not achieved until 4 weeks.
Treatment of chronic heart failure:
- Your treatment should be started and supervised under medical control.
- Your doctor will start your treatment with 1.25 mg per day (half a 2.5 mg tablet). The dose can be increased after 1-2 weeks to 2.5 mg per day (one 2.5 mg tablet or half a 5 mg tablet), then to 5 mg per day (two 2.5 mg tablets or one 5 mg tablet), and finally to 10 mg per day (four 2.5 mg tablets or two 5 mg tablets) to achieve the optimal dose. Your doctor will prescribe the correct dose at each time, and you must follow their instructions exactly.
- The maximum recommended dose is 10 mg per day.
- The start of treatment and each dose increase will be done under the supervision of an experienced doctor during a period of 2 hours.
- Your doctor will reduce your dose if necessary.
- Do not stop treatment abruptly, as this could worsen your heart failure.
- Patients with severe kidney problems should not take this medicine.
- Take the medicine once a day, preferably at the same time each day.
- Your doctor may decide to combine your tablets with other medicines to treat your disease.
If your doctor has told you to take ¼ (1.25 mg - a quarter) or ½ (2.5 mg - half) of a tablet daily, follow the instructions below to split the cross-scored Nebivolol Aurovitas 5 mg tablets.
- Place the tablets on a flat, hard surface (e.g., table or countertop), with the cross-score facing up.
- Break the tablet by pressing it with your index fingers on both hands, placed on either side of one of the scores (diagrams 1 and 2).
- Proceed in the same way to split the half-tablet into a quarter (diagrams 3 and 4).
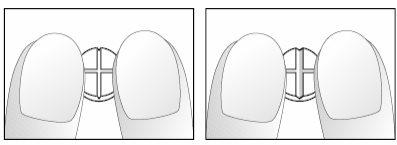
Diagrams 1 and 2: Easy breaking of the 5 mg nebivolol tablet, cross-scored, into a half.
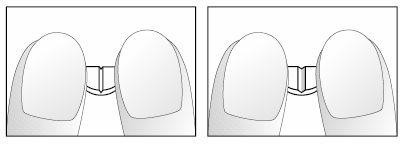
Diagrams 3 and 4: Easy breaking of the half 5 mg nebivolol tablet, cross-scored, into a quarter.
Use in children and adolescents
Nebivolol is not recommended in children and adolescents.
If you take more Nebivolol Aurovitas than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediatelyor call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
The most frequent symptoms and signs of a nebivolol overdose are a very slow heart rate (bradycardia), low blood pressure with possible fainting (hypotension), difficulty breathing like asthma (bronchospasm), and acute heart failure.
You can take activated charcoal (available at your pharmacy) while waiting for your doctor to arrive.
If you forget to take Nebivolol Aurovitas
If you forget to take your medicine, but remember to take it within a short period, take the next tablet as usual. However, if there is a long delay (e.g., several hours), such that it is close to the next dose, skip the missed dose and take the next scheduled doseat the usual time. Do not take a double dose to make up for the missed dose. You should try to avoid repeatedly forgetting to take your medicine.
If you stop taking Nebivolol Aurovitas
Always consult your doctor before stopping treatment with nebivolol, whether you are taking it for high blood pressure or chronic heart failure.
Do not stop treatment abruptly, as this could temporarily worsen your heart failure. If it is necessary to stop treatment for chronic heart failure, the daily dose should be gradually reduced, starting by halving the dose at weekly intervals.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
When Nebivolol Aurovitas is used to treat high blood pressure, the possible side effects are:
Frequent (may affect up to 1 in 10 people):
- Headache.
- Dizziness.
- Fatigue.
- Unusual itching or tingling sensation.
- Diarrhea.
- Constipation.
- Nausea.
- Breathing difficulties.
- Sweating of hands and feet.
Uncommon (may affect up to 1 in 100 people):
- Slow heart rate or other heart disorders.
- Low blood pressure.
- Pain when walking similar to a cramp.
- Abnormal vision.
- Impotence.
- Feeling of depression.
- Difficulty digesting (dyspepsia), stomach or intestinal gas, vomiting.
- Skin rash, itching.
- Breathing difficulties like asthma, due to a sudden contraction of the muscles surrounding the airways (bronchospasm).
- Nightmares.
Rare (may affect up to 1 in 10,000 people):
- Fainting.
- Worsening of psoriasis (a skin disease characterized by scaly pink patches).
The following side effects have been reported in isolated cases during treatment with this medicine:
- Allergic reactions throughout the body, with generalized skin rashes (hypersensitivity reactions).
- Sudden swelling, especially around the lips, eyelids, and/or tongue, which may be accompanied by acute breathing difficulties (angioedema).
- Skin rash characterized by pink, raised patches that itch, of allergic or non-allergic origin (urticaria).
In a clinical study for chronic heart failure, the following side effects were observed:
Very common (may affect more than 1 in 10 people):
- Slow heart rate.
- Dizziness.
Frequent (may affect up to 1 in 10 people):
- Worsening of heart failure.
- Low blood pressure (such as a feeling of dizziness when standing up quickly).
- Intolerance to this medicine.
- Mild cardiac conduction disorder affecting the heart rhythm (first-degree atrioventricular block).
- Swelling of the lower limbs (such as swelling of the ankles).
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nebivolol Aurovitas
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date is the last day of the month indicated.
This medicine does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the SIGRE collection point at your pharmacy. Ask your pharmacist how to dispose of the containers and any unused medicines. This will help protect the environment.
6. Packaging Contents and Additional Information
Composition ofNebivolol Aurovitas
- The active ingredient is nebivolol.
Each tablet contains 5.45 mg of nebivolol hydrochloride, equivalent to 5 mg of nebivolol.
- The other ingredients are lactose monohydrate, corn starch, sodium croscarmellose, hypromellose 15 cp, polysorbate 80, anhydrous colloidal silica, microcrystalline cellulose (PH-102), and magnesium stearate.
Appearance of the Product and Packaging Contents
Uncoated tablets, white to off-white in color, round (diameter of 9.1 mm), biconvex, marked with N L 5 and scored in a cross shape on one side of the tablet and smooth on the other side.
The tablet can be divided into equal doses (halves and quarters).
Nebivolol Aurovitas is available in blister packs and HDPE bottles with polypropylene closures.
Packaging Sizes:
Blister Packs:14, 28, 30, 50, 60, 90, and 100 tablets.
HDPE Bottles:250 tablets.
Only some packaging sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Aurovitas Spain, S.A.U.
Avda. de Burgos, 16-D
28036 Madrid
Spain
Manufacturer:
APL Swift Services (Malta) Limited
HF26, Hal Far Industrial Estate, Hal Far
Birzebbugia, BBG 3000
Malta
Generis Farmacêutica, S.A.
Rua João de Deus, 19
Amadora 2700-487
Portugal
This Medicinal Product is Authorized in the Member States of the European Economic Area under the Following Names:
Germany: Nebivolol PUREN 5 mg Tabletten
Belgium: Nebivolol AB 5 mg tabletten
Nebivolol AB 5 mg comprimés
Nebivolol AB 5 mg, Tabletten
Spain: Nebivolol Aurovitas 5 mg comprimidos EFG
Italy: Nebivololo Aurobindo Italia
Netherlands: Nebivolol Aurobindo 5 mg, tabletten
Poland: Nebivolol Aurovitas
Portugal: Nebivolol Generis
Czech Republic: Nebivolol Aurovitas 5 mg tablety
Date of the Last Revision of this Leaflet: April 2023
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
- Country of registration
- Average pharmacy price7.87 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NEBIVOLOL AUROVITAS 5 mg TABLETSDosage form: TABLET, 10 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 2.5 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription requiredDosage form: TABLET, 5 mgActive substance: nebivololManufacturer: Glenmark Arzneimittel GmbhPrescription required
Online doctors for NEBIVOLOL AUROVITAS 5 mg TABLETS
Discuss questions about NEBIVOLOL AUROVITAS 5 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











