
МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ

Спросите врача о рецепте на МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ

Инструкция по применению МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ
Введение
ПРОСПЕКТ: ИНФОРМАЦИЯ ДЛЯ ПОЛЬЗОВАТЕЛЯ
МИНИТРАН 15 мг/24 ч трансдермальные пластыри
Тринитрат глицерина
Прочитайте весь листок внимательно перед началом использования лекарства, поскольку он содержит важную информацию для вас.
- Сохраните этот листок, поскольку вам может потребоваться перечитать его.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и его не следует давать другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этом листке. См. раздел 4.
Содержание листка:
- Что такое МИНИТРАН и для чего он используется
- Что вам нужно знать перед началом использования МИНИТРАНА
- Как использовать МИНИТРАН
- Возможные побочные эффекты
5 Хранение МИНИТРАНА
- Содержание упаковки и дополнительная информация
1. Что такое МИНИТРАН и для чего он используется
Пластыри МИНИТРАНА являются трансдермальными системами, состоящими из тонкой и прозрачной пленки полиэтилена низкой плотности, покрытой адгезивной матрицей, содержащей тринитрат глицерина. Матрица контролирует скорость высвобождения активного вещества, обеспечивая постоянное высвобождение тринитрата глицерина в течение 24 часов. Пластырь покрыт защитной пленкой из полиэстера, которую необходимо удалить перед использованием.
МИНИТРАН показан для профилактики стенокардии напряжения и покоя, связанной с коронарной недостаточностью.
2. Что вам нужно знать перед началом использования МИНИТРАНА
Не используйте МИНИТРАН
- Если вы аллергичны к тринитрату глицерина и органическим нитратам или к любому другому компоненту этого лекарства (указанному в разделе 6).
- Если у вас есть сердечная недостаточность, вызванная обструкцией клапанов (сужением клапанов сердца) или воспалением перикарда, сдавливающего сердце.
- Если у вас есть заболевания, связанные с повышением внутричерепного давления (например, кровоизлияние в мозг или черепно-мозговая травма) или повышением внутриглазного давления (глаукома).
- В случаях снижения кровообращения, связанного с очень низким артериальным давлением (например, при шоке).
- Если у вас очень низкое артериальное давление (ниже 90 мм рт. ст.).
- В случаях тяжелой гиповолемии (если вы недавно потеряли много крови).
- Если вы принимаете лекарства группы ингибиторов фосфодиэстеразы 5, такие как силденафил, тадалафил, варденафил (лекарства, используемые для лечения импотенции или легочной гипертензии). Эти лекарства никогда не должны приниматься одновременно с МИНИТРАНОМ.
- Если вы принимаете лекарства с риоцигуатом, стимулятором растворимой гуанилатциклазы.
Для получения более подробной информации проконсультируйтесь с вашим врачом или фармацевтом.
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом использования МИНИТРАНА.
- МИНИТРАН должен использоваться под строгим медицинским контролем в случаях недавнего инфаркта миокарда или сердечной недостаточности (неспособности сердца перекачивать достаточно крови для остального тела).
- Сообщите вашему врачу, если у вас есть или были:
- любые сердечные заболевания или расстройства кровеносных сосудов, отличные от стенокардии,
- любые легочные заболевания. У пациентов со стенокардией, инфарктом миокарда или ишемией мозга могут возникать нарушения дыхательных путей,
- анемия.
- У пациентов со стенокардией, вызванной утолщением сердца (гипертрофической кардиомиопатией), лечение МИНИТРАНОМ может ухудшить состояние.
- МИНИТРАН не показан для лечения острых приступов стенокардии (боли в груди), требующих сочетания нитратов быстрого действия перорально.
- В случае прекращения лечения доза и частота применения пластыря МИНИТРАНА должны быть постепенно снижены в течение 4-6 недель, чтобы избежать реакций отмены этого класса vasodilatadores. Если вы начинаете другое лечение стенокардии, в течение некоторого времени вам может потребоваться использовать оба лекарства.
- Продукт может вызывать ортостатическую гипотензию, особенно у тревожных пациентов, поэтому необходимо предупредить пациентов об этом риске, чтобы избежать внезапных изменений положения тела.
- Возможно развитие привыкания к лекарству или другим нитратам. По этой причине врач может рекомендовать применять МИНИТРАН ежедневно с интервалом без пластыря 8-12 часов в день.
Использование МИНИТРАНА с другими лекарствами
Сообщите вашему врачу или фармацевту, если вы используете, недавно использовали или можете использовать любое другое лекарство.
Пациенты, получающие лечение МИНИТРАНОМ, никогда не должны принимать лекарства ингибиторов фосфодиэстеразы 5 (ПДЭ5) такие как силденафил, варденафил или тадалафил (лекарства, используемые для лечения импотенции и легочной гипертензии). Для получения более подробной информации проконсультируйтесь с вашим врачом или фармацевтом.
Необходимо избегать одновременного лечения с риоцигуатом, стимулятором растворимой гуанилатциклазы, поскольку одновременное использование может вызвать гипотензию (см. «Не используйте МИНИТРАН»).
Желательно, чтобы врач знал, если вы проходите лечение другими лекарствами, поскольку они могут увеличить или уменьшить эффект снижения артериального давления МИНИТРАНА.
Сочетанное применение с другими vasodilatadores, такими как алкоголь и некоторые лекарства (блокаторы кальция, бета-блокаторы, диуретики, антигипертензивные, трициклические антидепрессанты, седативные средства), должно проводиться с осторожностью, чтобы избежать добавления эффектов.
Нестероидные противовоспалительные лекарства (НПВЛ), кроме ацетилсалициловой кислоты (аспирина), могут уменьшить действие МИНИТРАНА.
Одновременное использование МИНИТРАНА с дигидроэрготамином (лекарством, используемым для лечения мигрени), может увеличить количество дигидроэрготамина в крови. Это важно для пациентов с коронарной недостаточностью, поскольку дигидроэрготамин противодействует действию МИНИТРАНА.
Сочетанное применение МИНИТРАНА с амифостином и ацетилсалициловой кислотой может усилить гипотензивные эффекты МИНИТРАНА.
Влияние на диагностические тесты
Тринитрат глицерина может влиять на измерение некоторых клинических анализов (катехоламинов и ваниломанделиевой кислоты в моче, увеличивая их экскрецию).
Использование МИНИТРАНА с продуктами питания и напитками
Не рекомендуется употреблять алкогольные напитки во время лечения.
Беременность, лактация и фертильность
Если вы беременны или в период лактации, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием любого лекарства.
МИНИТРАН должен использоваться с осторожностью во время беременности, особенно в течение первых трех месяцев беременности. В этих обстоятельствах лекарство является подходящим только в случае необходимости и под прямым контролем врача. Если вы регулярно используете это лекарство и становитсяе беременной, немедленно сообщите об этом вашему врачу.
Если вы в период лактации, вам необходимо предупредить врача, поскольку он должен решить, необходимо ли прекратить лактацию или прекратить лечение, учитывая пользу лактации для ребенка и пользу лечения для матери.
Вождение и использование машин
МИНИТРАН может вызывать гипотензию и головокружение, особенно в начале лечения. Если вы заметите любой из этих эффектов, избегайте вождения транспортных средств или использования машин.
3. Как использовать МИНИТРАН
Следуйте точно инструкциям по применению этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого лекарства.
Ответ на нитраты варьируется в зависимости от индивидуума и должен быть назначен в каждом случае минимально эффективная доза. Поскольку количество тринитрата глицерина, высвобождаемого пластырем МИНИТРАНА, является постоянным, доза, вводимая пациенту, зависит только от площади контакта пластыря.
Как правило, рекомендуется начинать лечение с пластыря МИНИТРАНА 5 мг/24 ч в день. Если необходимо, и в зависимости от продемонстрированной толерантности, можно увеличить лечение до пластыря МИНИТРАНА 10 мг/24 ч или даже МИНИТРАНА 15 мг/24 ч в день.
Применение может быть в течение непрерывного периода 24 часов или интермиттирующе, включая интервал без пластыря 8-12 часов (обычно ночью).
Использование у детей и подростков:Безопасность и эффективность у детей и подростков моложе 18 лет не установлены. Поэтому его использование не рекомендуется.
Использование у пожилых людей:Пожилые пациенты обычно более чувствительны к гипотензивным эффектам (снижению артериального давления).
Инструкции по правильному применению
Каждый пластырь МИНИТРАНА находится в небольшой защитной упаковке. Адгезивная зона покрыта защитной пленкой, которую необходимо удалить непосредственно перед применением на кожу.
Нанесите пластырь МИНИТРАН на кожу в чистую, сухую, здоровую зону (без остатков кремов).
Правильная процедура применения следующая:
- Откройте упаковку, разорвав ее по метке, и извлеките пластырь изнутри.
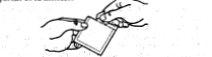
- Слегка согните пластырь с точечными метками, обращенными к вам, потяните за язычок и выбросьте защитную пленку.

- Нанесите адгезивную поверхность на верхнюю часть руки или на грудь. Аккуратно отделите другую точечную часть защитной пленки.

- Нажмите на пластырь, чтобы обеспечить его хорошее положение.
Удалите пластырь МИНИТРАН через 24 часа, если врач не рекомендует иное. Пластыри МИНИТРАНА являются одноразовыми. Они должны храниться вне досягаемости детей.
Нанесите новый пластырь МИНИТРАН, следуя описанной методике. Этот новый пластырь должен быть нанесен на другую зону, чем предыдущий, например, на противоположную сторону груди.
Не наносите систему на одну и ту же зону в течение нескольких дней подряд. Пластырь МИНИТРАНА легко прикрепляется к коже и не отрывается при купании, душе или физических упражнениях.
Если вы использовали больше МИНИТРАНА, чем следует
Высокие дозы тринитрата глицерина могут в некоторых случаях вызвать быстрое снижение артериального давления и привести к шоку, тахикардии, метгемоглобинемии, цианозу, коме и судорогам.
Из-за контролируемого высвобождения тринитрата глицерина с помощью МИНИТРАНА риск передозировки очень низок.
Эффект нитрата МИНИТРАНА может быть быстро устранен путем простого удаления пластыря (пластырей).
Любое снижение артериального давления и симптомы коллапса можно лечить, поднимая ноги и, если необходимо, обматывая их.
Тяжелая метгемоглобинемия (повышение метгемоглобина в крови) может быть лечена инъекцией метилтионина или толония.
В случае передозировки или случайного приема немедленно обратитесь в медицинское учреждение или позвоните в Центр токсикологической информации. Телефон (91) 562 04 20, указав лекарство и количество, принятое внутрь.
Если вы забыли использовать МИНИТРАН
Не используйте двойную дозу, чтобы компенсировать пропущенные дозы
Если вы прекратили лечение МИНИТРАНОМ
В случае прекращения лечения доза и частота применения пластыря должны быть постепенно снижены в течение 4-6 недель, чтобы избежать реакций отмены этого класса vasodilatadores.
Если у вас есть какие-либо другие вопросы об использовании этого продукта, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, МИНИТРАН может вызывать побочные эффекты, хотя не все люди их испытывают.
Толерантность к тринитрату глицерина обычно хорошая. Следующие побочные эффекты могут возникать и исчезать во время лечения.
Очень часто (могут возникать более чем у 1 из 10 человек): тошнота и рвота.
Часто (могут возникать до 1 из 10 человек): головная боль, которая обычно проходит через несколько дней и может быть лечена легкими обезболивающими. В случаях особенно интенсивной головной боли может быть необходимо снизить дозу или прекратить лечение.
Не часто (могут возникать до 1 из 100 человек): дерматит (воспаление кожи). раздражение кожи (зуд, жжение и легкое покраснение), которое обычно проходит через несколько часов после удаления пластыря. Если вы испытываете общую реакцию кожи, которая охватывает большую зону, сообщите об этом вашему врачу.
Редко (могут возникать до 1 из 1000 человек): покраснение лица. Головокружение, особенно при быстром подъеме, поэтому рекомендуется делать это медленно и, если вы чувствуете головокружение, садиться или лежать. Снижение артериального давления. Обморок. Ускорение сердечного ритма.
Очень редко (могут возникать до 1 из 10 000 человек): головокружение
Неизвестно (количество пациентов, на которых влияет побочный эффект, не может быть рассчитано с помощью имеющейся информации): сердцебиение (необычное ощущение сердечного ритма), высыпание на коже и обморок.
Важно, чтобы вы сообщили об этом вашему врачу, поскольку он может потребоваться снизить дозу или прекратить лечение, по крайней мере временно.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этом листке. Вы также можете сообщить об этом напрямую через систему фармакологического мониторинга www.notificaRAM.es
Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение МИНИТРАНА
Не храните выше 25°C. Храните в прохладном и сухом месте.
Держите вне досягаемости и поля зрения детей.
Не используйте это лекарство после истечения срока годности, указанного на упаковке. Срок годности является последним днем месяца, указанного на упаковке.
Лекарства не должны выбрасываться в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в пункт сбора SIGRE в аптеке. Если у вас есть сомнения, проконсультируйтесь с вашим фармацевтом о том, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав МИНИТРАНА 15 мг/24 ч
- Активное вещество - тринитрат глицерина, 54 мг на пластырь. Среднее количество, высвобождаемое в течение 24 часов, составляет 15 мг.
- Другие компоненты:
Кополиэтилен/акриламид: этиловый олеат; монолаурат глицерина.
Поддержка: овальная пленка полиэтилена низкой плотности; пленка полиэстера, покрытая силиконом с одной стороны.
Внешний вид продукта и содержание упаковки
МИНИТРАН 15 мг - это трансдермальные пластыри, состоящие из тонкой и прозрачной пленки полиэтилена низкой плотности, покрытой адгезивной матрицей, содержащей тринитрат глицерина. Матрица контролирует скорость высвобождения активного вещества. Пластырь покрыт защитной пленкой из полиэстера, которую необходимо удалить перед использованием.
Каждый пластырь имеет площадь 20 см² и выпускается в упаковках по 7 и 30 пластырей.
Владелец разрешения на продажу и ответственный за производство
Владелец разрешения на продажу:
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Дублин 15
Дублин
Ирландия
Ответственный за производство:
Meda Pharma GmbH & Co. KG
Benzstrasse 1
61352 Бад-Хомбург
Германия
или
Mylan Hungary Kft.
Mylan улица 1
2900 Комаром
Венгрия
Вы можете получить более подробную информацию о этом лекарстве, обратившись к местному представителю владельца разрешения на продажу:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Мадрид
Испания
Дата последнего обновления этого листка:октябрь 2017
Подробная и актуальная информация о этом лекарстве доступна на сайте Агентства по лекарствам и медицинским изделиям (AEMPS) https://www.aemps.gob.es

Сколько стоит МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в Испании в 2025 году?
Средняя цена на МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в декабрь, 2025 года составляет около 23.14 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках23.14 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 37,4 мг нитроглицеринаАктивное вещество: глицерил тринитратПроизводитель: Merus Labs Luxco Ii S.À.R.L.Требуется рецептФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 18,7 мг нитроглицеринаАктивное вещество: глицерил тринитратПроизводитель: Merus Labs Luxco Ii S.À.R.L.Требуется рецептФорма выпуска: ТРАНСДЕРМАЛЬНЫЙ ПЛАСТЫРЬ, 31,37 мгАктивное вещество: глицерил тринитратПроизводитель: Casen Recordati S.L.Требуется рецепт
Аналоги МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в Польша
Аналог МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ в Украина
Врачи онлайн по МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на МИНИТРАН 15 мг/24 ч ТРАНСДЕРМАЛЬНЫЕ ПЛАСТЫРИ – по решению врача и с учетом местных правил.










