LIDBREE 42 mg/mL INTRAUTERINE GEL


How to use LIDBREE 42 mg/mL INTRAUTERINE GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET
Package Leaflet: Information for the patient
Lidbree 42 mg/ml intrauterine gel
lidocaine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Lidbree and what is it used for
- What you need to know before you use Lidbree
- How to use Lidbree
- Possible side effects
5 Conservation of Lidbree
- Contents of the pack and further information
1. What is Lidbree and what is it used for
Lidbree is an anesthetic gel used to prevent pain from gynecological procedures, such as the placement of contraceptive devices in the uterus and biopsy sampling for laboratory evaluation in gynecological examinations, in adults and adolescents from 15 years of age. It contains the active ingredient lidocaine, a local anesthetic of the amide type (which numbs the parts of the body to which it is applied).
How does Lidbree work?
After applying the gel, it takes 2 to 5 minutes before the genital area (mucosa) is numb. It has been shown that the gel reduces pain during gynecological procedures and up to at least 30 minutes after the procedure. After 1 hour, the analgesic effect disappears.
2. What you need to know before you use Lidbree
Do not use Lidbree
If you are allergic to lidocaine or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Exclusive use by endocervical and intrauterine route. After using the gel for the placement of an intrauterine contraceptive (intrauterine device, IUD), in cases of difficult insertion, bleeding and/or exceptional pain may occur. In these cases, a physical examination and an ultrasound should be performed immediately to rule out a perforation of the uterus (matrix) or cervix (cervix). The average is 1 perforation per 1,000 IUD insertion procedures.
Inform the healthcare professional who will administer Lidbree to you:
- if you have a heart rhythm disorder (partial or complete cardiac conduction block) as local anesthetics may affect it;
- if you are being treated for a heart rhythm disorder (with potassium channel blockers or class III antiarrhythmics (e.g., amiodarone)) because cardiac effects may increase;
- if you have a condition called acute porphyria (a family condition related to one of the proteins in the blood). Lidocaine may cause porphyria attacks and should only be prescribed in patients with acute porphyria in urgent or necessary cases;
- if you have a poor general state of health.
Children and adolescents
This medicine should not be administered to children under 15 years of age due to the risk of side effects caused by high concentrations of lidocaine in the blood.
Other medicines and Lidbree
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine that contains lidocaine or medicines to treat irregular heart rhythm (antiarrhythmics, such as mexiletine or class III antiarrhythmics like amiodarone), as their effects on the heart will be added to those of lidocaine.
Pregnancy, breastfeeding, and fertility
Based on long-term experience, it is unknown whether lidocaine produces adverse effects in the newborn.
Lidocaine can be secreted in breast milk, but in such small amounts that there is generally no risk of affecting the newborn. Therefore, breastfeeding can continue in case of treatment with Lidbree.
The effect of lidocaine on fertility is unknown.
Driving and using machines
Lidbree has a negligible influence on the ability to drive and use machines.
Lidbree contains macrogolglycerol ricinoleate (polyoxyethylated castor oil) and butylhydroxytoluene
Macrogolglycerol ricinoleate may cause severe allergic reactions.
This medicine may cause local skin reactions (such as contact dermatitis) or eye and mucous membrane irritation because it contains butylhydroxytoluene (E321).
3. How to use Lidbree
Your doctor or nurse will apply the anesthetic gel step by step, starting from the entrance of the uterus.
Use inadolescents
Adolescents with low weight, below 30 kg body weight, should receive a reduced dose.
If you use more Lidbree than you should
With the recommended doses, the following effects are not expected to occur; however, inform your doctor or nurse immediately if you experience numbness of the lips or tongue, dizziness, ringing in the ear (tinnitus), or have difficulty speaking or seeing correctly (visual disturbances), as they may be the first symptoms of high concentrations of lidocaine in the blood. In some cases, muscle spasms or tremors (tremors) or interruptions of breathing (apnea) may occur; in that case, your doctor or nurse should quickly ensure that you are breathing correctly (respiratory support) and administer anticonvulsants.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used or ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects experienced after using Lidbree for the insertion of intrauterine contraceptives (matrix) are similar to those that occur in the same process without the application of Lidbree.
Possible side effects are:
- Very common(more than 1 in 10 people): nausea (feeling unwell).
- Common(may affect up to 1 in 10 people): dizziness, headache, unpleasant sensations in the abdomen.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es By reporting side effects, you can help provide more information on the safety of this medicine.
5. Conservation of Lidbree
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date (month-year) that appears on the carton and on the syringe. The expiry date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Lidbree
- The active ingredient is lidocaine. Each ml of intrauterine gel contains 42 mg of lidocaine.
- The other components are:
- Macrogolglycerol ricinoleate (polyoxyethylated castor oil)
- Poloxamer (contains butylhydroxytoluene (E 321))
- Sodium ascorbate (E 301)
- Hydrochloric acid for pH adjustment
- Sodium hydroxide for pH adjustment
- Water for injectable preparations
Appearance of the product and contents of the pack
The product is a sterile, transparent or almost transparent, slightly brownish-yellow viscous liquid intrauterine gel at room temperature, containing 42 mg/ml of lidocaine. The formulation shows a reversible temperature-dependent gelification, it is a gel at body temperature (thermogelification). Lidbree 42 mg/ml intrauterine gel is presented in a 10 ml pre-filled sterile syringe (cycloolefin copolymer) with a bromobutyl rubber tip cap and a plunger rod, packaged in a blister pack with the plunger rod. The syringe is graduated in ml. A sterile applicator (polypropylene) with a Luer lock adapter compatible with the pre-filled syringe is provided in a separate bag within the carton. 8.5 ml of gel can be extracted from the applicator.
Pack size: 1 x 10 ml intrauterine gel in pre-filled syringe.
Symbols on the Lidbree applicator label
|
|
|
|
|
Catalog number | Batch code | Do not use if the packaging is damaged | Do not reuse | CE marking |
|
|
|
| |
Manufacturer | Expiry date | Sterilized by irradiation | Consult the instructions for use |
Marketing authorization holder and manufacturer
Marketing authorization holder
Gedeon Richter Plc.
Gyömroi út 19-21.
Budapest H-1103
Hungary
Manufacturers:
Recipharm Karlskoga AB
Björkbornsvägen 5
SE-691 33 Karlskoga
Sweden
Gedeon Richter Plc.
Gyömroi út 19-21.
Budapest H-1103
Hungary
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Gedeon Richter Ibérica, S.A.
Sabino Arana, 28 - 4º 2ª
08028 Barcelona
Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria, Belgium, Bulgaria, Cyprus, Czech Republic, Germany, Denmark, Estonia, Greece, Spain, Finland, France, Croatia, Hungary, Ireland, Iceland, Italy, Luxembourg, Latvia, Lithuania, Malta, Norway, Poland, Portugal, United Kingdom, Romania, Sweden, Slovenia, Slovakia: Lidbree
Date of the last revision of thisleaflet:June2020
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
----------------------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Exclusive use by endocervical and intrauterine route.
After using Lidbree, in cases of difficult insertion and/or exceptional pain or bleeding during or after insertion, a physical examination and an ultrasound should be performed immediately to rule out a perforation of the uterus (matrix) or cervix (cervix), as with effective topical anesthesia, the patient may not react with pain in case of perforation.
Thermogelifying formulation:Lidbree is a local anesthetic, viscous liquid, thermogelifying, without preservatives. The formulation forms a gel when the temperature increases to body temperature, adhering to the mucous tissue of the cervical canal and uterus (minimizing losses that would occur with a liquid formulation).
Method of application and dosage
Lidbree should be administered in a liquid state. If a gel forms, place it in the refrigerator until it becomes liquid again. The visible air bubble in the syringe moves when the syringe is tilted.
Prepare the sterile applicator included in the packaging by following the indicated steps and apply the medicine:
- Check the appearance of the syringe while tilting it. The air bubble will move when tilting the syringe when the product is in a liquid state, ready to use. If the air bubble does not move, it is because a gel has formed; in that case, you should place the syringe in the refrigerator until it becomes liquid again.
- Connect the plunger rod and the applicator to the syringe, ensuring they are well engaged.

- Remove the air bubble and fill the applicator with gel by carefully pushing the syringe plunger.
- Use the centimeter scale on the applicator to measure the amount of Lidbree.
Once the applicator is in place, 8.5 ml of gel can be administered from the syringe. One ml contains 42 mg of lidocaine. Apply the gel step by step (1 to 3) as illustrated in the figure.
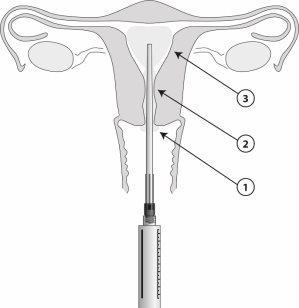
Cervical procedures
- Using the sterile applicator, apply 2 to 3 ml of the gel in a thick layer on the exocervix.
- Using the applicator, administer 3 ml into the cervical canal 5 minutes before the start of the procedure.
Intrauterine procedures
- Using the sterile applicator, apply 1 to 2 ml of the gel on the anterior lip of the exocervix.
- Using the applicator, administer 2 to 3 ml into the cervical canal. Wait 2 minutes for the effect to begin in the internal canal.
- Then, insert the applicator into the uterine cavity and introduce 3 to 5 ml of the gel, 5 minutes before the procedure. The applicator is marked with a centimeter scale. A smaller volume can be administered, e.g., in nulliparous patients, if the patient experiences discomfort before finishing administering the entire volume.
A single intrauterine dose should not exceed 10 ml. Discard the unused contents.
Pediatric population from 15 years of age.
In adolescents with low weight, body weight below 30 kg, the dose should be reduced proportionally; the single dose should not exceed the recommended maximum parenteral dose (6 mg/kg of lidocaine hydrochloride, which corresponds to 5.2 mg/kg of lidocaine base in Lidbree, i.e., 1.2 ml per 10 kg of body weight). In adolescents with a body weight of 30 kg, the maximum dose of Lidbree is 3.6 ml in total.
Duration of the effect
It has been shown that the gel reduces pain during gynecological procedures and up to at least 30 minutes after the procedure. After 1 hour, the analgesic effect has disappeared.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LIDBREE 42 mg/mL INTRAUTERINE GELDosage form: DRESSING, 700 mgActive substance: lidocaineManufacturer: Grünenthal Pharma S.A.Prescription requiredDosage form: GEL/PASTE/ORAL LIQUID, 20 mg/gActive substance: lidocaineManufacturer: Chemische Fabrik Kreussler & Co GmbhPrescription not requiredDosage form: GEL, 20 mg/mlActive substance: lidocaineManufacturer: Farco-Pharma GmbhPrescription required
Online doctors for LIDBREE 42 mg/mL INTRAUTERINE GEL
Discuss questions about LIDBREE 42 mg/mL INTRAUTERINE GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions