LAMBDALINA 40 mg/g CREMA

Cómo usar LAMBDALINA 40 mg/g CREMA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
LAMBDALINA 40 mg/g crema
Lidocaina
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es LAMBDALINA y para qué se utiliza
- Qué necesita saber antes de empezar a usar LAMBDALINA
- Cómo usar LAMBDALINA
- Posibles efectos adversos
- Conservación de LAMBDALINA
- Contenido del envase e información adicional
1. Qué es LAMBDALINA y para qué se utiliza
LAMBDALINA 40 mg/g crema contiene lidocaína y es un medicamento que induce la pérdida de sensibilidad local (anestesia local) de la piel.
LAMBDALINA se usa para desensibilizar la piel en relación a la inserción de agujas (por ej. previo a análisis de sangre).
2. Qué necesita saber antes de empezar a usar LAMBDALINA
No use LAMBDALINA
- si es alérgico a lidocaína, o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si es alérgico (hipersensible) a anestésicos locales, soja o cacahuetes.
- en niños prematuros (nacidos antes de la 37ª semana de gestación).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar LAMBDALINA:
- en caso de intervenciones quirúrgicas en el canal auditivo o en el oído interno ya que existe riesgo de dañar el oído interno.
- si padece alguna enfermedad grave por ej. enfermedad cardiaca o insuficiencia hepática grave.
- si toma medicamentos para alteraciones del ritmo cardíaco (ver Uso de otros medicamentos)
- cerca de los ojos, ya que lidocaína puede causar irritación ocular. Además, con la pérdida de reflejos protectores, se puede dar irritación corneal o rasguños. Si LAMBDALINA entra en contacto con los ojos, lavarlos con agua tibia inmediatamente y protegerlos hasta que vuelva la sensación.
- si está a punto de recibir una vacunación con una vacuna viva (por ej. la vacuna de la tuberculosis). Las vacunas no se deben administrar en áreas donde LAMBDALINA se haya aplicado ya que el efecto de la vacuna puede verse afectado.
Por favor, informe a su médico si le afecta alguna de las situaciones anteriormente mencionadas.
LAMBDALINA debe utilizarse únicamente en piel no dañada (no en membranas mucosas, heridas o eczema).
Otros medicamentos y LAMBDALINA
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Es especialmente importante para su médico saber si usted ha sido ya tratado con alguno de los medicamentos que se mencionan a continuación, ya que el riesgo de efectos adversos puede verse incrementado:
- lidocaína a altas dosis por otras razones.
- otros anestésicos locales
- medicamentos para arritmias (alteraciones del ritmo cardíaco) por ej. tocainida, mexiletina y amiodarona.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
El uso ocasional de LAMBDALINA durante el embarazo es poco probable que cause efectos dañinos en el feto.
LAMBDALINA pasa a leche materna pero es poco probable que afecte al bebé durante la lactancia.
Conducción y uso de máquinas
LAMBDALINA no tiene influencia conocida sobre la capacidad para conducir y utilizar máquinas.
LAMBDALINA contiene propilenglicol, alcohol bencílico, lecitina de soja hidrogenada y polisorbato 80.Este medicamento contiene 75 mg de propilenglicol por cada gramo de crema. El propilenglicol puede provocar irritación en la piel.
Lambdalina contiene lecitina de soja hidrogenada. Si es alérgico a los cacahuetes o la soja, no use este medicamento.
Este medicamento contiene 15 mg de alcohol bencílico por cada gramo de crema. El alcohol bencílico puede provocar reacciones alérgicas y puede provocar irritación local moderada.
Este medicamento contiene polisorbato 80 que puede causar reacciones alérgicas.
3. Cómo usar LAMBDALINA
Siga exactamente las instrucciones de administración de LAMBDALINA indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es:
Niños de6 a12 años:
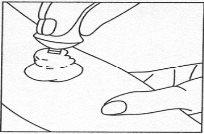
La dosis única a aplicar es de 2-3g. El tiempo de aplicación recomendado, es decir, el tiempo que la crema debe estar en la piel, es de 60 minutos, pero nunca superior a dos horas.
Adolescentes mayores de 12 años y adultos:
La dosis única a aplicar es de 2-3g. La dosis máxima diaria es de 5 gramos. El tiempo de aplicación recomendado es de 60 minutos, pero nunca superior a dos horas.
Niños de2 a6 años:
Dado que no hay suficiente experiencia, LAMBDALINA no se recomienda en este grupo de edad.
Niños de0 a2 años:
Dado que no hay experiencia, LAMBDALINA no debe usarse en este grupo de edad.
1 gramo de crema corresponde aproximadamente a una longitud de 2,5 cm.
|
|
|
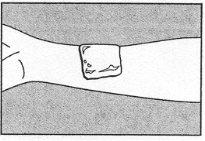
Se recomienda un vendaje para evitar que la crema se desprenda accidentalmente antes de que finalice el tiempo de aplicación. |
|
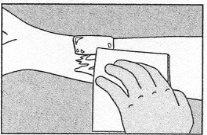
Cerrar el tubo tras su uso. |
Si tiene la impresión de que el efecto de LAMBDALINA es demasiado fuerte o débil, por favor consulte con su médico o farmacéutico.
Si usa más LAMBDALINA del que debe
La sobredosis es poco probable con un uso normal en piel no dañada.
Si ha usado más LAMBDALINA del que debiera, o si un niño ha ingerido el medicamento por accidente o se le ha aplicado demasiado en la piel, por favor, contacte con su médico o con el hospital para tener una valoración del riesgo y consejo.
En caso de sobredosis o ingestión accidental, consultar al Servicio de Información Toxicológica, teléfono 91562 04 20.
Si tiene cualquier otra pregunta sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Poco frecuentes (1 a10 de 1000 personas tratadas):
Reacciones cutáneas locales (palidez, enrojecimiento, picor y escozor) en el área tratada. Los síntomas son normalmente transitorios y leves.
Raras(1 a10 de 10000 personas tratadas):
Eczema alérgico de contacto.
Muy raras (menos de 1 de 10000 personas tratadas)
Reacciones alérgicas (en casos graves, shock anafiláctico)
Deje de utilizar LAMBDALINA y contacte inmediatamente con su médico si tiene cualquiera de los siguientes síntomas (shock anafiláctico)
- Hinchazón de cara, lengua o garganta
- Dificultad al tragar
- Urticaria y problemas respiratorios.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto.
También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de LAMBDALINA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el tubo después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30º C.
Tras la apertura del tubo, la caducidad es de 6 meses.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e Información adicional
Composición de LAMBDALINA
- El principio activo es lidocaína. 1 gramo de crema contiene 40 mg de lidocaína.
- Los demás componentes son agua purificada, propilenglicol, lecitina de soja hidrogenada, alcohol bencílico, polisorbato 80, carbómero 940, trolamina, colesterol.
Aspecto del producto y contenido del envase
LAMBDALINA es una crema blanca a amarillenta.
Presentaciones: 5 g, 10 g, 20 g, 30 g, 40 g y 50 g en tubos de aluminio. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación.
Titular:
ISDIN, SA
Provençals 33
08019 Barcelona
España
Fabricante:
Medinfar Manufacturing S.A.
Parque Industrial Armando Martins Tavares
Rua Outeiro da Armada, 5
Condeixa-a-Nova
3150-194 Sebal
Portugal
Fecha de la última revisión de este prospecto: mayo 2025
- País de registro
- Precio medio en farmacia15.64 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LAMBDALINA 40 mg/g CREMAForma farmacéutica: APÓSITO, 700 mgPrincipio activo: LidocainaFabricante: Grünenthal Pharma S.A.Requiere recetaForma farmacéutica: GEL/PASTA/LIQUIDO BUCAL, 20 mg/gPrincipio activo: LidocainaFabricante: Chemische Fabrik Kreussler & Co GmbhNo requiere recetaForma farmacéutica: GEL, 20 mg/mlPrincipio activo: LidocainaFabricante: Farco-Pharma GmbhRequiere receta
Médicos online para LAMBDALINA 40 mg/g CREMA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LAMBDALINA 40 mg/g CREMA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes