ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце
Спросите врача о рецепте на ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце

Инструкция по применению ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце
Введение
Инструкция: информация для пользователя
Икатибант Ципла 30 мг раствор для инъекции в предварительно заполненном шприце EFG
Прочитайте внимательно всю инструкцию перед началом использования этого лекарства, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться прочитать ее снова.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Это лекарство было назначено только вам, и не передавайте его другим людям, даже если у них такие же симптомы, как у вас, поскольку оно может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции
- Что такое Икатибант Ципла и для чего он используется
- Что вам нужно знать перед началом использования Икатибанта Ципла
- Как использовать Икатибант Ципла
- Возможные побочные эффекты
- Хранение Икатибанта Ципла
- Содержание упаковки и дополнительная информация
1. Что такое Икатибант Ципла и для чего он используется
Икатибант Ципла содержит активное вещество икатибант.
Это лекарство используется для лечения симптомов наследственного ангиоэдемы (НА) у взрослых, подростков и детей старше 2 лет.
При НА увеличиваются концентрации вещества в крови, называемого брадикинином, что приводит к симптомам, таким как отек, боль, тошнота и диарея.
Икатибант блокирует действие брадикинина и, таким образом, замедляет прогрессию симптомов криза НА.
2. Что вам нужно знать перед началом использования Икатибанта Ципла
Не используйте Икатибант Ципла
- если вы аллергичны к икатибанту или любому другому компоненту этого лекарства (перечисленному в разделе 6).
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом перед началом использования Икатибанта Ципла:
- Если вы страдаете стенокардией (снижением кровотока к сердцу).
- Если вы недавно перенесли инсульт.
Побочные эффекты, связанные с икатибантом, подобны симптомам вашего заболевания. Немедленно проконсультируйтесь с вашим врачом, если заметите, что симптомы криза ухудшаются после введения этого лекарства.
Кроме того:
- Вы или ваш опекун должны научиться технике введения подкожных инъекций (под кожу) перед тем, как самостоятельно или с помощью опекуна вводить это лекарство.
- Немедленно после введения Икатибанта Ципла или после введения его опекуном во время ларингеальной кризы (обструкции верхних дыхательных путей), вы должны обратиться за медицинской помощью в медицинское учреждение.
- Если ваши симптомы не исчезают после введения одной инъекции Икатибанта Ципла, вам следует проконсультироваться с врачом о введении дополнительных инъекций этого лекарства. У взрослых пациентов можно вводить до 2 дополнительных инъекций в течение 24 часов.
Дети и подростки
Не рекомендуется использовать это лекарство у детей младше 2 лет или у детей, вес которых меньше 12 кг, поскольку оно не было изучено у этих пациентов.
Другие лекарства и Икатибант Ципла
Сообщите вашему врачу, если вы принимаете, недавно принимали или можете принимать любое другое лекарство.
Не известны взаимодействия Икатибанта Ципла с другими лекарствами. Если вы принимаете любое лекарство, являющееся ингибитором ангиотензин-превращающего фермента (АПФ) (например, каптоприл, эналаприл, рамиприл, квинаприл, лизиноприл) для снижения артериального давления или по любой другой причине, сообщите вашему врачу перед использованием этого лекарства.
Беременность и лактация
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом перед началом использования этого лекарства.
Если вы кормите грудью, не кормите вашего ребенка в течение 12 часов после последнего введения этого лекарства.
Вождение и использование машин
Не управляйте транспортными средствами и не используйте машины, если вы чувствуете усталость или головокружение в результате кризы НА или после использования этого лекарства.
Икатибант Ципла содержит натрий
Это лекарство содержит менее 1 ммоль (23 миллиграмма) натрия на шприц, поэтому оно считается практически "без натрия".
3. Как использовать Икатибант Ципла
Следуйте точно инструкциям по введению этого лекарства, указанным вашим врачом. В случае сомнений проконсультируйтесь с вашим врачом снова.
Если вам никогда ранее не вводили Икатибант Ципла, первая доза всегда должна быть введена медицинским персоналом или медсестрой. Врач выпишет вам направление, когда считает, что можно безопасно отправить вас домой.
После обсуждения с вашим врачом или медсестрой и после обучения технике подкожных инъекций (под кожу) вы сами или человек, ухаживающий за вами, можете вводить это лекарство, если у вас возникла криза НА.
Важно вводить Икатибант Ципла подкожно (под кожу) как можно скорее после обнаружения кризы ангиоэдемы. Медицинский персонал научит вас и человека, ухаживающего за вами, безопасному введению этого лекарства, следуя инструкциям в инструкции.
Когда и с какой частотой следует использовать Икатибант Ципла?
Ваш врач определил точную дозу этого лекарства и скажет, с какой частотой его следует использовать.
Взрослые
- Рекомендуемая доза Икатибанта Ципла составляет одну инъекцию (3 мл, 30 мг), введенную подкожно (под кожу) как можно скорее после обнаружения кризы ангиоэдемы (например, с увеличением кожного отека, особенно на лице и шее, или увеличением боли в животе).
- Если вы не заметите улучшения симптомов после 6 часов, вам следует проконсультироваться с врачом о введении дополнительных инъекций Икатибанта Ципла. У взрослых пациентов можно вводить до 2 дополнительных инъекций в течение 24 часов.
- Не вводите более 3 инъекций в течение 24 часов, и если вам требуется более 8 инъекций в месяц, проконсультируйтесь с вашим врачом.
Дети и подростки от 2 до 17 лет
- Рекомендуемая доза этого лекарства составляет одну инъекцию 1 мл до максимальной дозы 3 мл в зависимости от веса тела, введенную подкожно (под кожу) как можно скорее после обнаружения симптомов кризы ангиоэдемы (например, увеличения кожного отека, особенно на лице и шее, или увеличения боли в животе).
- Проконсультируйтесь с разделом инструкций по использованию, чтобы увидеть дозу, которую необходимо вводить.
- Если вы не уверены в дозе, которую необходимо вводить, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой.
- Если ваши симптомы ухудшаются или не улучшаются, немедленно проконсультируйтесь с врачом.
Как следует вводить Икатибант Ципла?
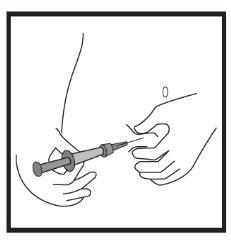
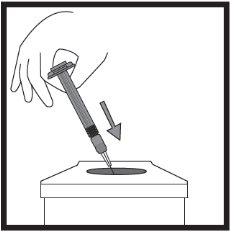
Икатибант Ципла вводится подкожно (под кожу). Каждый шприц следует использовать только один раз.
Это лекарство вводится с помощью короткой иглы в жировую ткань под кожей живота (брюшка).
Если у вас есть какие-либо другие вопросы о использовании этого лекарства, проконсультируйтесь с вашим врачом или фармацевтом.
Следующие инструкции шаг за шагом предназначены для:
- самовведения (взрослые)
- введения опекуном или медицинским работником для взрослых, подростков или детей старше 2 лет (весом не менее 12 кг).
Инструкции включают следующие основные шаги:
- Общая информация
2а) Подготовка шприца для детей и подростков (2-17 лет) весом 65 кг или менее
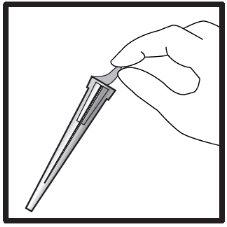
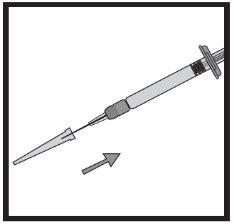
2б) Подготовка шприца и иглы для инъекции (все пациенты)
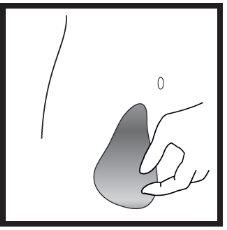
- Подготовка места инъекции
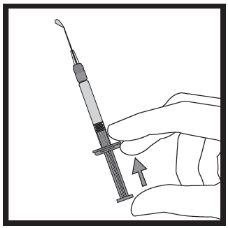
- Введение раствора
- Утилизация материалов инъекции
Инструкции шаг за шагом для инъекции
| ||||||||||
| ||||||||||
2а) Подготовка шприца для детей и подростков (2-17 лет) весом 65 кг или менее: | ||||||||||
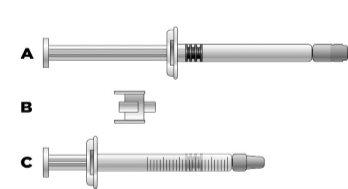
Важная информация для медицинских работников и опекунов: Когда доза меньше 30 мг (3 мл), для извлечения необходимой дозы требуется следующее оборудование (см. информацию ниже):
Объем инъекции, необходимый в мл, следует подготовить в пустом graduированном шприце 3 мл (см. таблицу ниже). Таблица 1: Позологическая таблица для детей и подростков
Пациенты, вес которых превышает 65 кг, используют весь содержимое предварительно заполненного шприца (3 мл).
Перелить раствор икатибанта в graduированный шприц:
| ||||||||||
Если в graduированном шприце есть воздух:
| ||||||||||
|
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их. Почти все пациенты, получающие Икатибант Ципла, замечают реакцию в месте инъекции (такую как раздражение кожи, воспаление, боль, зуд, покраснение кожи и жжение). Эти эффекты обычно легкие и улучшаются без необходимости дополнительного лечения.
Сообщите вашему врачу немедленно, если вы заметите, что симптомы кризы ухудшаются после введения этого лекарства.
Очень частые(могут возникать у более 1 из 10 человек):
- Дополнительные реакции в месте инъекции (чувство давления, синяк, снижение чувствительности и/или онемение, увеличение кожной сыпи с зудом и жаром).
Частые(могут возникать у до 1 из 10 человек):
- Тошнота
- Головная боль
- Головокружение
- Лихорадка
- Зуд
- Сыпь
- Покраснение кожи
- Аномальные пробы функции печени
Частота не известна(не может быть оценена из доступных данных):
- Крапивница (уртикария)
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, проконсультируйтесь с вашим врачом или фармацевтом, даже если это возможные побочные эффекты, которые не указаны в этой инструкции. Вы также можете сообщить об этом напрямую через Испанскую систему фармакологической безопасности лекарств для человека: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете внести свой вклад в предоставление более полной информации о безопасности этого лекарства.
5. Хранение Икатибанта Ципла
Храните это лекарство вне поля зрения и досягаемости детей.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке после "CAD". Дата истечения срока годности - последний день месяца, указанного.
Не храните при температуре выше 25°C. Не замораживайте.
Не используйте это лекарство, если вы заметите, что упаковка шприца или иглы повреждена или если вы заметите видимые признаки ухудшения; например, если раствор мутный, содержит плавающие частицы или если цвет раствора изменился.
Лекарства не должны выбрасываться в канализацию или в мусор. Спросите вашего фармацевта, как утилизировать упаковку и лекарства, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержимое упаковки и дополнительная информация
Состав Икатибанто Ципла
- Активное вещество - икатибант. Каждая предварительно заполненная шприц содержит 30 миллиграммов икатибанто (в форме ацетата). Каждый мл раствора содержит 10 мг икатибанто
- Другие компоненты - хлорид натрия, уксусная кислота, гидроксид натрия (для регулирования pH) и вода (см. раздел 2).
Внешний вид продукта и содержимое упаковки
Икатибанто Ципла представлен в виде прозрачной и бесцветной инъекционной раствора в стеклянной предварительно заполненной шприце объемом 3 мл. Упаковка содержит гиподермическую иглу.
Икатибанто Ципла выпускается в индивидуальной упаковке, содержащей одну предварительно заполненную шприцу с иглой, или в упаковке, содержащей три предварительно заполненные шприцы с тремя иглами.
Возможно, что будут продаваться только некоторые размеры упаковок.
Владелец разрешения на маркетинг и производитель
Владелец разрешения на маркетинг
Сипла Европа НВ
Де Кейзерлей 58-60, ящик 19,
2018 Антверпен, Бельгия
Производитель
Фармадокс Хелскеа Лтд.
КВ20А Кордин Индастриал Парк
Паола ПЛА 3000
Мальта
или
Еврофинс Прокси Лабораториз (PRX)
Архимедесвег 25 2333 СМ Лейден
Нидерланды
Местный представитель
Сипла Европа НВ, филиал в Испании,
К/Гусман эль Буэно, 133 Эдиф Британия 28003 Мадрид, Испания
Это лекарство разрешено в государствах-членах Европейского экономического пространства под следующими названиями:
Германия | Икатибант Ципла 30 мг инъекционный раствор, т.е. готовый шприц |
Дания | Икатибант Ципла |
Испания | Икатибанто Ципла 30 мг раствор для инъекций в предварительно заполненной шприце ЕФГ |
Норвегия | Икатибант Ципла |
Дата последнего обзора этой инструкции:Сентябрь 2021
Подробная и актуальная информация о этом лекарстве доступна на сайте Испанского агентства по лекарствам и медицинским изделиям (АЕМПС) (http://www.aemps.gob.es/)
- Страна регистрации
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприцеФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 30 мгАктивное вещество: ИкатибантПроизводитель: Takeda Pharmaceuticals International Ag Ireland BranchТребуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 30 мгАктивное вещество: ИкатибантПроизводитель: Accord Healthcare S.L.U.Требуется рецептФорма выпуска: ИНЪЕКЦИОННЫЙ РАСТВОР, 30 мгАктивное вещество: ИкатибантПроизводитель: Laboratoire AguettantТребуется рецепт
Аналоги ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце в Польша
Аналог ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце в Украина
Врачи онлайн по ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ИКАТИБАНТО CIPLA 30 мг Раствор для инъекций в предварительно заполненном шприце – по решению врача и с учетом местных правил.




 Если вы не уверены в объеме раствора, который необходимо извлечь, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой
Если вы не уверены в объеме раствора, который необходимо извлечь, проконсультируйтесь с вашим врачом, фармацевтом или медсестрой Избегайте прикосновения к концам коннектора и кончикам шприца, чтобы не произошло загрязнение.
Избегайте прикосновения к концам коннектора и кончикам шприца, чтобы не произошло загрязнение.