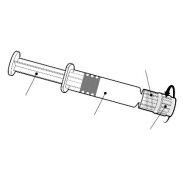
FLUARIX TETRA Injectable Suspension in Pre-filled Syringe

How to use FLUARIX TETRA Injectable Suspension in Pre-filled Syringe
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fluarix Tetra injectable suspension in a pre-filled syringe
Influenza vaccine (split virion, inactivated)
This package leaflet has been written to be read by the person who has been prescribed the vaccine. However, the vaccine can be administered to adolescents and children, so you may be reading this for your child.
Read all of this package leaflet carefully before you or your child receive this vaccine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This vaccine has been prescribed to you or your child, do not pass it on to others.
- If you or your child experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Fluarix Tetra and what is it used for
- What you need to know before you receive Fluarix Tetra
- How Fluarix Tetra is administered
- Possible side effects
5
Storage of Fluarix Tetra
- Contents of the pack and further information
1. What is Fluarix Tetra and what is it used for
Fluarix Tetra is a vaccine that helps protect you against influenza, particularly in people with a high risk of complications. The use of Fluarix Tetra should be based on official recommendations.
When Fluarix Tetra is administered to a person, their immune system (the body's natural defense system) will produce its own protection (antibodies) against the disease. None of the vaccine components can cause influenza.
Influenza is a disease that spreads quickly and is caused by different strains that can change each year. This is why you may need to be vaccinated every year. The period with the highest risk of contracting influenza is during the cold months, between October and March. If you were not vaccinated in the autumn, it is recommended that you do so before spring, as you are at risk of infection until then. Your doctor will recommend the best time to be vaccinated.
Fluarix Tetra will protect you against the four virus strains from approximately 2 to 3 weeks after injection.
The incubation period of influenza is a few days, so if you are exposed to influenza immediately before or after vaccination, you can still develop the disease.
The vaccine will not protect you against the common cold, even though some of the symptoms are similar to those of influenza.
2. What you need to know before you receive Fluarix Tetra
To ensure that Fluarix Tetra is suitable for you, it is important that you inform your doctor or pharmacist if any of the following points apply to you. If there is anything you do not understand, ask your doctor or pharmacist to explain.
Do not use Fluarix Tetra
- If you are allergic to the active substances or to any of the other components of this medicine (listed in section 6) or to any component that may be present in very small amounts such as eggs (ovoalbumin or chicken proteins), formaldehyde, gentamicin sulfate, or sodium desoxycholate.
- If you have any disease accompanied by high fever or acute infection, vaccination should be delayed until you have recovered.
Warnings and precautions
Consult your doctor or pharmacist before receiving Fluarix Tetra:
Your doctor will decide if you should receive the vaccine.
Before or after any injection, fainting (especially in adolescents) may occur, so you should inform your doctor or nurse if you have fainted after receiving an injection in the past.
Like all vaccines, Fluarix Tetra may not completely protect all vaccinated people.
Use of Fluarix Tetra with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Fluarix Tetra can be administered at the same time as other vaccines if they are injected into different limbs.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Your doctor or pharmacist will decide if you should receive Fluarix Tetra. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Fluarix Tetra has no or negligible influence on the ability to drive and use machines.
Fluarix Tetra contains sodium
This medicine contains less than 23 mg of sodium (1 mmol) per dose; it is essentially "sodium-free".
Fluarix Tetra contains potassium
This medicine contains less than 39 mg (1 mmol) of potassium per dose, so it is considered essentially "potassium-free".
3. How Fluarix Tetra is administered
Dose
Adults will receive a dose of 0.5 ml.
Use in children:
Children from 6 months onwards will receive a dose of 0.5 ml.
If your child is under 9 years old and has not been previously vaccinated against influenza, a second dose should be administered after an interval of at least 4 weeks.
Method and/or route of administration
Your doctor will administer the recommended dose of the vaccine by injection into the muscle.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
The following side effects have been observed during clinical trials:
Side effects that occurred in children between 6 and 36 months of age
Very common(may occur with more than 1 in 10 doses of the vaccine): loss of appetite, irritability, sleepiness, pain and/or redness at the injection site.
Common(may occur with up to 1 in 10 doses of the vaccine): fever, swelling at the injection site.
Side effects that occurred in children between 3 and 6 years of age
Very common(may occur with more than 1 in 10 doses of the vaccine): pain and/or redness and/or swelling at the injection site, irritability.
Common(may occur with up to 1 in 10 doses of the vaccine): loss of appetite, sleepiness, fever.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): skin rash, itching at the injection site.
Side effects that occurred in children between 6 and 18 years of age
Very common(may occur with more than 1 in 10 doses of the vaccine): muscle pain, pain and/or redness and/or swelling at the injection site, fatigue.
Common(may occur with up to 1 in 10 doses of the vaccine): nausea, diarrhea, vomiting, stomach pain, headache, joint pain, chills, fever.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): skin rash, itching at the injection site.
Side effects that occurred in adults ≥18 years of age
Very common(may occur with more than 1 in 10 doses of the vaccine): pain at the injection site, fatigue, muscle pain (myalgia).
Common(may occur with up to 1 in 10 doses of the vaccine): headache, nausea, diarrhea, vomiting, stomach pain, joint pain (arthralgia), fever, chills, redness and/or swelling at the injection site.
Uncommon(may occur with up to 1 in 100 doses of the vaccine): bruising (hematoma), itching (pruritus) around the area where the vaccine is injected, dizziness.
In addition, the side effects that occurred during studies in subjects from 3 years of age with Fluarix (trivalent influenza vaccine) were:
Common(may occur with up to 1 in 10 doses of the vaccine): hardening (induration) around the area where the vaccine is injected, sweating.
These reactions usually disappear within 1-2 days without treatment.
In addition to the side effects mentioned above, the following side effects occurred during routine use of Fluarix and/or Fluarix Tetra:
Rare(may occur with up to 1 in 1000 doses of the vaccine):
- Allergic reactions:
- leading to a medical emergency due to failure of the circulatory system to provide adequate blood flow to the various organs of the body (shock),
- swelling, most noticeable in the head and neck, including the face, lips, tongue, throat, or any other part of the body (angioedema).
- Skin reactions that can spread throughout the body, including itching of the skin (pruritus, urticaria) and redness (erythema) of the skin.
- Neurological disorders that can cause stiffness in the neck, confusion, numbness, pain, and weakness in the limbs, loss of balance, loss of reflexes, partial or total paralysis of the body (encephalomyelitis, neuritis, Guillain-Barré syndrome).
- Temporary swelling of the lymph nodes in the neck, armpits, or groin (transient lymphadenopathy).
- Symptoms similar to those of influenza, general malaise.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Agency's website, www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluarix Tetra
Keep this vaccine out of the sight and reach of children.
Do not use this vaccine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month shown.
Store in a refrigerator (between 2°C and 8°C).
Do not freeze.
Store in the outer carton to protect from light.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Fluarix Tetra
The active substances are: influenza virus (split virion, inactivated) of the following strains*:
Strain similar to A/Victoria/4897/2022 (H1N1)pdm09:
(IVR-238) derived from A/Victoria/4897/202215 micrograms of HA**
Strain similar to A/Croatia/10136RV/2023 (H3N2):
(X-425A) derived from A/Croatia/10136RV/202315 micrograms of HA**
Strain similar to B/Austria/1359417/2021:
(BVR-26) derived from B/Austria/1359417/202115 micrograms of HA**
Strain similar to B/Phuket/3073/2013:
(wild-type) derived from B/Phuket/3073/201315 micrograms of HA**
per 0.5 ml dose
- propagated in embryonated chicken eggs from healthy chicken flocks
**hemagglutinin
This vaccine complies with the World Health Organization (WHO) recommendation for the Northern Hemisphere and with the European Union recommendation for the 2025/2026 campaign.
The other components are: sodium chloride, disodium phosphate dodecahydrate, potassium dihydrogen phosphate, potassium chloride, magnesium chloride hexahydrate, alpha-tocopherol hydrogen succinate, polysorbate 80, octoxinol 10, and water for injections.
Appearance of the product and pack contents
Fluarix Tetra is an injectable suspension in a pre-filled syringe.
Fluarix Tetra is available in a pre-filled syringe with or without separate needles; pack sizes of 1 and 10.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
GlaxoSmithKline Biologicals s.a.
Rue de l’Institut 89
B-1330 Rixensart
Belgium
Manufacturer
GlaxoSmithKline Biologicals NL der SmithKline Beecham Pharma GmbH & Co. KG
Zirkusstrasse 40
D-01069 Dresden
Germany
Local representative
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel.: +34 900 202 700
This medicine is authorized in the Member States of the European Economic Area under the following names:
Member State | Name |
Austria, Bulgaria, Cyprus, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, Greece, Hungary, Ireland, Iceland, Italy, Latvia, Lithuania, Malta, Norway, Netherlands, Poland, Portugal, Czech Republic, Sweden | Fluarix Tetra |
Belgium, Luxembourg | Alpharix-Tetra |
Germany | Influsplit Tetra |
France | FluarixTetra |
Date of last revision of this package leaflet:07/2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es)
--------------------------------------------------------------------------------------------------------------------This information is intended only for healthcare professionals:
As with all injectable vaccines, adequate medical treatment and supervision should be available to deal with any allergic reactions that may occur after administration of the vaccine.
Immunization should be carried out by intramuscular injection.
Fluarix Tetra should never be administered intravascularly.
Fluarix Tetra can be administered at the same time as other vaccines. Immunization should be carried out in different limbs.
Allow the vaccine to reach room temperature before use.
Shake before use. Inspect visually before administration.
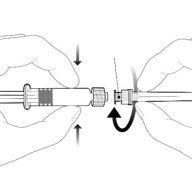
Instructions for the pre-filled syringe
| Hold the syringe by the body, not by the plunger. Remove the syringe cap by twisting it in a counter-clockwise direction. |
| To insert the needle, attach the base to the luer-lock adapter and twist it a quarter turn in a clockwise direction until it clicks. Do not pull the plunger out of the syringe body. If this happens, do not administer the vaccine. |
Disposal of waste
Disposal of unused medicine and all materials that have come into contact with it should be done in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUARIX TETRA Injectable Suspension in Pre-filled SyringeDosage form: INJECTABLE, 3.75 microgramsActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Glaxosmithkline BiologicalsPrescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription requiredDosage form: INJECTABLE, 60 micrograms of HAActive substance: influenza, inactivated, split virus or surface antigenManufacturer: Sanofi Winthrop IndustriePrescription required
Online doctors for FLUARIX TETRA Injectable Suspension in Pre-filled Syringe
Discuss questions about FLUARIX TETRA Injectable Suspension in Pre-filled Syringe, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions