
ACTAIR 300 IR COMPRIMIDOS SUBLINGUALES

Cómo usar ACTAIR 300 IR COMPRIMIDOS SUBLINGUALES
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Actair 300IR comprimidos sublinguales
Para uso en adolescentes y adultos (de 12 a 65años)
Extractos alergénicos estandarizados de ácaros del polvo doméstico
(Dermatophagoides pteronyssinusy Dermatophagoides farinae)
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Actair y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Actair
- Cómo tomar Actair
- Posibles efectos adversos
- Conservación de Actair
- Contenido del envase e información adicional
1. Qué es Actair y para qué se utiliza
Este medicamento contiene extractos alergénicos de ácaros del polvo doméstico.
Este medicamento se utiliza para tratar la rinitis alérgica (inflamación de la mucosa de la nariz) en adolescentes (12-17 años) y adultos. Este medicamento actúa aumentando la tolerancia inmunitaria a los ácaros del polvo doméstico (es decir, la capacidad del organismo para afrontar su presencia). Puede que no note una mejoría hasta que haya tomado el tratamiento durante 3 meses.
Antes de iniciar el tratamiento, un médico debe diagnosticarle la alergia mediante las pruebas cutáneas o los análisis de sangre apropiados.
La primera dosis de este medicamento se debe tomar bajo supervisión médica. Deberá permanecer en observación médica durante al menos media hora después de tomar el comprimido. Se trata de una precaución para observar su sensibilidad al medicamento. También le dará la oportunidad de comentar posibles efectos adversos a su médico.
Este medicamento debe recetarlo un médico con experiencia en el tratamiento de alergias.
2. Qué necesita saber antes de empezar a tomar Actair
No tome Actair
- Si es alérgico a alguno de los excipientes (demás componentes) de este medicamento (incluidos en la sección 6).
- Si sufre de asma grave y/o inestable o ha sufrido una exacerbación grave del asma en los últimos 3 meses.
- Si el médico determina que su volumen espiratorio forzado en un segundo [VEF1] es inferior al 80 %.
- Si tiene una enfermedad que afecta al sistema inmunitario, si toma medicamentos supresores del sistema inmunitario o si tiene cáncer.
- Si tiene úlceras o infecciones bucales. El médico le recomendará retrasar el comienzo del tratamiento o interrumpirlo hasta que tenga la boca curada.
No empiece a tomar este medicamento si está embarazada.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar este medicamento:
- Si sufre síntomas alérgicos graves, como dificultad para tragar o respirar, cambios en la voz, hipotensión (tensión arterial baja) o siente un nudo en la garganta. Detenga el tratamiento y póngase en contacto con el médico de inmediato.
- Si ha sufrido una reacción alérgica grave a un medicamento con extractos alergénicos con anterioridad.
- Si sus síntomas del asma empeoran más de lo normal. Detenga el tratamiento y póngase en contacto con el médico de inmediato.
- Si tiene una enfermedad cardiovascular.
- Si toma un bloqueante β-adrenérgico (una clase de medicamentos que suele recetarse para tratar enfermedades cardíacas y la tensión arterial alta y que también está presente en algunos colirios y pomadas).
- Si recibe tratamiento para la depresión con antidepresivos tricíclicos o inhibidores de la monoaminoxidasa (IMAO) o para la enfermedad de Parkinson con inhibidores de la catecol-O-metiltransferasa (COMT).
- Si va a someterse a una intervención quirúrgica bucal o a una extracción dental, interrumpa temporalmente el tratamiento con este medicamento hasta que haya cicatrizado por completo.
- Si sufre ardor de estómago o dificultad para tragar. En ese caso, contacte con su médico.
- Si tiene una enfermedad autoinmune en remisión.
Informe a su médico:
- De cualquier enfermedad que haya tenido recientemente;
- De los antecedentes personales o familiares de cualquier enfermedad que afecte al sistema inmunitario;
- Si su enfermedad alérgica ha empeorado recientemente.
No se debe interrumpir la toma de medicamentos de control y/o de alivio del asma sin el consejo de su médico, ya que puede empeorar los síntomas del asma.
Durante el tratamiento es de esperar que se produzcan algunas reacciones alérgicas localizadas de intensidad leve o moderada. Si las reacciones son graves, hable con el médico para determinar si necesita algún medicamento antialérgico como antihistamínicos.
Niños y adolescentes
Este medicamento se utiliza para tratar la rinitis alérgica en adolescentes (12-17 años). Este medicamento no se debe utilizar en niños menores de 12 años.
Otros medicamentos y Actair
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento, incluidos los medicamentos sin receta. Si está tomando otros medicamentos para la alergia, como antihistamínicos, medicamentos para el asma o esteroides o un medicamento que bloquee una sustancia denominada inmunoglobulina E (IgE), por ejemplo, omalizumab, hable con su médico sobre si debe seguir tomándolos. Si deja de tomar los medicamentos para la alergia, es posible que sufra más efectos secundarios durante el tratamiento con este medicamento.
Uso de Actair con alimentos y bebidas
No consuma alimentos ni bebidas en los 5 minutos después de tomar este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No hay experiencia sobre el uso de este medicamento durante el embarazo. Por lo tanto, no debe empezar el tratamiento con este medicamento si está embarazada. Si se queda embarazada mientras toma este medicamento, consulte a su médico sobre si es adecuado continuar el tratamiento.
No hay experiencia sobre el uso de este medicamento durante la lactancia materna. No obstante, no se prevén efectos sobre los lactantes. Consulte con su médico para saber si es adecuado continuar el tratamiento con este medicamento durante la lactancia materna.
Conducción y uso de máquinas
No se han observado efectos de este medicamento sobre la capacidad para conducir o usar máquinas.
Actair contiene lactosa
Este medicamento contiene lactosa. Si su médico le ha indicado que padece una intolerancia a algunos azúcares, cpóngase en contacto con él antes de tomar este medicamento.
Actair contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Actair
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Se recomienda tomar el primer comprimido bajo supervisión médica. Su médico le indicará durante cuánto tiempo debe tomar este medicamento.
El tratamiento empieza con una fase de inicio, es decir, la dosis se aumenta de manera progresiva hasta alcanzar la dosis de mantenimiento de 300 IR. El IR (Índice de Reactividad) expresa la actividad.
Tratamiento de mantenimiento
La dosis es de 300 IR (un comprimido) al día.
Uso enadolescentes
La pauta de administración en adolescentes es la misma que en adultos.
Tome este medicamento como se indica a continuación:
- Saque un comprimido del envase presionando el comprimido contra la lámina.
- Tome el comprimido durante el día, con la boca vacía.
- Ponga el comprimido debajo de la lengua, deje que se disuelva por completo y luego trague.
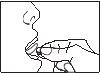
- No coma ni beba durante al menos 5 minutos.
- Lávese las manos después de manipular el comprimido.
Si toma más Actair del que debe
Si toma más este medicamento del que debe, puede sufrir síntomas alérgicos como síntomas localizados en la boca y la garganta. Si padece síntomas graves, contacte de inmediato con un médico o un hospital.
Si olvidó tomar Actair
Si ha olvidado tomar un comprimido, tómelo más tarde durante el día. No tome una dosis doble para compensar las dosis olvidadas. Si no ha tomado este medicamento durante más de 7 días, contacte con su médico antes de volver a tomar este medicamento.
Si interrumpe el tratamiento con Actair
Si no toma este medicamento según las indicaciones del médico, puede que no obtenga los efectos beneficiosos del tratamiento. Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos pueden ser una respuesta alérgica al alérgeno con el que se está tratando. La mayoría de los efectos adversos duran de minutos a horas después de tomar el medicamento y la mayor parte de ellos desaparecerán cuando haya estado en tratamiento durante 1 a 3 meses.
Deje de tomar este medicamento y contacte de inmediato con el médico o el hospital si sufre cualquiera de estos síntomas:
- Rápida hinchazón de la cara, la boca, la garganta o la piel
- Dificultad para tragar
- Dificultad para respirar
- Cambios en la voz
- Hipotensión (tensión arterial baja)
- Sensación de nudo en la garganta (como hinchazón)
- Urticaria y picor en la piel
Otros posibles efectos adversos:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- Hinchazón o picor en la boca
- Irritación de garganta
- Picor en el oído
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Picor en los ojos
- Hinchazón o picor en los labios o la lengua
- Quemazón u hormigueo en la boca, inflamación o llagas en la boca, úlcera bucal
- Alteración del gusto
- Molestias o dolor en la boca o la garganta
- Inflamación de garganta, dificultad para tragar
- Tos
- Dificultad para respirar
- Dolor en el pecho
- Dolor de estómago, indigestión, náuseas, diarrea
- Prurito
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Enrojecimiento e inflamación de los ojos, ojos hinchados, lagrimeo
- Dolor en el oído u hormigueo
- Vértigo, mareos
- Dolor de cabeza
- Malestar general o fatiga
- Rinitis (estornudos, goteo o picor nasal, obstrucción nasal)
- Hemorragia nasal
- Resfriado común
- Inflamación de los labios y la lengua
- Alteraciones en la boca como quemazón, entumecimiento, candidiasis bucal, problemas salivales
- Hinchazón del paladar
- Hinchazón de la cara
- Sequedad de la boca o la garganta, sed
- Ampollas en la boca o la garganta, hinchazón de la boca y la garganta debido a frutas o verduras
- Alteraciones en la garganta como quemazón/hormigueo o tirantez, ronquera, sensación de nudo en la garganta, molestias o hinchazón en la parte posterior de la garganta
- Asma, disnea, respiración sibilante
- Molestias en el pecho
- Dolor en el esófago, inflamación del esófago o el estómago, ardor de estómago
- Vómitos
- Gastroenteritis
- Hinchazón localizada, edema subcutáneo
- Erupción, irritación cutánea, urticaria
- Ansiedad
- Sensación de hormigueo o pinchazos
- Resultados anormales en los análisis de sangre
Raros (pueden afectar hasta 1 de cada 1.000 personas):
- Inflamación de los párpados, espasmos de los párpados, irritación ocular
- Bloqueo del oído, zumbido de oídos
- Molestias nasales, obstrucción de los senos paranasales
- Inflamación de encías, sangrado en la boca
- Halitosis, eructos
- Dolor al tragar
- Irritación de la laringe
- Respiración rápida
- Entumecimiento en la garganta
- Alergia estacional
- Bronquitis
- Dolor de mamas
- Palpitaciones, latido rápido
- Inflamación del esófago
- Defecación frecuente, intestino irritable, gases
- Irritabilidad, alteración de la atención, embotamiento, somnolencia, trastornos del habla, temblores
- Ampollas, enrojecimiento de la piel, reacción cutánea aguda, lesiones por rascado
- Molestias o contracciones musculares
- Incontinencia imperiosa
Si le preocupa algún efecto secundario, consulte a su médico para que decida si necesita tomar algún medicamento, como antihistamínicos, para su alivio.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ACTAIR
Este medicamento no requiere condiciones especiales de conservación.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Actair
- El principio activo es un extracto alergénico estandarizado de los ácaros del polvo doméstico Dermatophagoides pteronyssinusy Dermatophagoides farinae. Un comprimido sublingual contiene 300 IR.
El IR (índice de reactividad) expresa la actividad.
- Los demás componentes son sílice coloidal anhidra, croscarmelosa sódica, lactosa monohidrato, estearato de magnesio, manitol (E-421) y celulosa microcristalina.
Aspecto del producto y contenido del envase
Comprimido sublingual.
Los comprimidos de 300 IR son de color blanco a beige, redondos y biconvexos, con motas marrones y con "SAC" grabado en una cara y "300" grabado en la otra.
Los comprimidos se suministran en blísteres de aluminio sellados con una lámina de aluminio separable y dentro de una caja de cartón.
Tamaños de envases:
Envase de 30 comprimidos sublinguales
Envase de 90 comprimidos sublinguales
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Stallergenes
Rue Alexis de Tocqueville, 6
92160 Antony
Francia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Stallergenes Ibérica S.A.
Llacuna, 22 – 2º 1ª
08005 – Barcelona
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeoy en el Reino Unido (Irlanda del Norte)con los siguientes nombres:
Austria Actair 300 IR Sublingualtabletten
Bélgica Orylmyte 300 IR comprimés sublinguaux
Bulgaria ?????? 300 IR ???????????? ????????
Croacia Orylmyte 300 IR sublingvalne tablete
República Checa, Polonia, Portugal, Rumanía ACTAIR
Dinamarca, Noruega, Suecia Aitmyte
Francia Orylmyte 300 IR comprimé sublingual
Alemania ORYLMYTE 300 IR
Irlanda, Reino Unido (Irlanda del Norte) ACTAIR 300 IR sublingual tablets
Italia, Luxemburgo ORYLMYTE
Países Bajos Actair 300 IR, tabletten voor sublinguaal gebruik
Eslovenia Actair 300 IR podjezicne tablete
Eslovaquia ACTAIR 300 IR sublingválne tablety
España Actair 300 IR comprimidos sublinguales
Fecha de la última revisión de este prospecto: Enero 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia98.82 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ACTAIR 300 IR COMPRIMIDOS SUBLINGUALESForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 12 SQ-HDMPrincipio activo: house dust mitesFabricante: Alk-Abello A/SRequiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 100 IR + 300 IRPrincipio activo: house dust mitesFabricante: StallergenesRequiere recetaForma farmacéutica: COMPRIMIDO SUBLINGUAL, 100 mgPrincipio activo: house dust mitesFabricante: StallergenesRequiere receta
Médicos online para ACTAIR 300 IR COMPRIMIDOS SUBLINGUALES
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ACTAIR 300 IR COMPRIMIDOS SUBLINGUALES, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















