VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA

Pergunte a um médico sobre a prescrição de VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA

Como usar VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA
Introdução
Bula:informação para o utilizador
Vaxigrip Tetra, suspensão injetável em seringa pré-carregada
Vacina antigripal tetravalente (vírus fracionados, inativados)
Leia todo o folheto informativo atentamente antes de si ou seu filho serem vacinados porque contém informações importantes para si ou seu filho.
- Conserva este folheto informativo, pois pode ter que relê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Esta vacina foi prescrita apenas para si ou seu filho e não deve dá-la a outras pessoas, mesmo que tenham os mesmos sintomas que você, pois pode prejudicá-las.
- Se você ou seu filho experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste folheto informativo. Ver seção 4.
Conteúdo do folheto informativo
- O que é Vaxigrip Tetra e para que é utilizado
- O que precisa saber antes de você ou seu filho começarem a usar Vaxigrip Tetra
- Como usar Vaxigrip Tetra
- Posíveis efeitos adversos
5 Conservação de Vaxigrip Tetra
- Conteúdo do envase e informações adicionais
1. O que é Vaxigrip Tetra e para que é utilizado
Vaxigrip Tetra é uma vacina.
Esta vacina é administrada a você ou seu filho a partir dos 6 meses de idade, está indicada para protegê-lo(a) contra a gripe.
Quando é injetada em uma pessoa, Vaxigrip Tetra, o sistema imunológico (o sistema de defesas naturais do corpo) produzirá proteção (anticorpos) contra a infecção.
Quando a vacina é administrada durante a gravidez, além de proteger a mulher grávida, também protege o seu bebê desde o nascimento até os 6 meses de idade, através da transmissão da proteção da mãe para o bebê durante a gravidez (ver também as seções 2 e 3).
Nenhum dos componentes da vacina pode causar gripe.
O uso de Vaxigrip Tetra deve basear-se nas recomendações oficiais.
A gripe é uma doença que pode se espalhar rapidamente e é causada por diferentes tipos de cepas que podem mudar a cada ano. Devido a essa mudança potencial nas cepas circulantes a cada ano, bem como a duração da proteção prevista pela vacina, a vacinação é recomendada todos os anos. Há um maior risco de contrair gripe durante os meses frios, entre outubro e março. Se você ou seu filho não se vacinaram no outono, é possível se vacinar até a primavera, pois você ou seu filho correm o risco de se infectar com a gripe durante esse período. O seu médico poderá recomendar a melhor data para a vacinação.
O objetivo de Vaxigrip Tetra é proteger você ou seu filho contra as quatro cepas do vírus contidas na vacina, após cerca de 2 a 3 semanas da injeção. O período de incubação da gripe é de alguns dias, de modo que, se você ou seu filho se expuser à gripe imediatamente antes ou após a vacinação, poderá desenvolver a doença.
A vacina não o(a) protegerá contra o resfriado comum, mesmo que alguns dos sintomas sejam semelhantes aos da gripe.
2. O que precisa saber antes de começar a usar Vaxigrip Tetra
Para garantir que Vaxigrip Tetra é adequado para você ou seu filho, é importante que consulte o seu médico ou farmacêutico se algum dos pontos descritos a seguir o(a) afetar. Se houver algo que você não entende, consulte o seu médico ou farmacêutico para que o esclareça.
Não use Vaxigrip Tetra
- Se você ou seu filho são alérgicos a:
- Os princípios ativos ou
- A qualquer um dos outros componentes desta vacina (enumerados na seção 6), ou
- A qualquer um dos componentes que podem estar presentes em quantidades mínimas, como ovos (ovoalbúmina ou proteínas de frango), neomicina, formaldeído ou octoxinol-9.
- Se você ou seu filho padecem de uma doença que se acompanha de febre alta ou moderada ou uma doença aguda, nesse caso, a vacinação será adiada até que se recuperem.
Advertências e precauções
Consulte o seu médico, farmacêutico ou enfermeiro antes de começar a usar Vaxigrip Tetra.
Consulte o seu médico antes de se vacinar se você ou seu filho têm:
- Uma resposta imunológica debilitada (imunodeficiência ou tratamento com medicamentos que afetem o sistema imunológico),
- Problemas de sangramento ou hematomas que se formam com facilidade.
O seu médico decidirá se você ou seu filho devem receber a vacina. Antes ou após qualquer injeção, pode ocorrer um desmaio (especialmente em adolescentes), por isso, informe o seu médico ou enfermeiro se você ou seu filho desmaiaram em ocasiões anteriores após a administração de uma injeção.
Como todas as vacinas, Vaxigrip Tetra pode não proteger totalmente todas as pessoas que se vacinam.
Nem todos os bebês menores de 6 meses de idade nascidos de mulheres grávidas vacinadas durante a gravidez estarão protegidos.
Se, por alguma razão, for realizado um exame de sangue em você ou seu filho nos dias seguintes à vacinação contra a gripe, por favor, informe o seu médico. Isso é porque foram observados resultados falsos positivos em testes sorológicos em alguns pacientes vacinados recentemente.
Crianças
Não se recomenda o uso de Vaxigrip Tetra em crianças menores de 6 meses de idade.
Uso de Vaxigrip Tetracomoutros medicamentos
Informar o seu médico ou farmacêutico se você ou seu filho estão utilizando, utilizaram recentemente ou possam ter que utilizar qualquer outro medicamento ou vacina.
- Vaxigrip Tetra pode ser administrado ao mesmo tempo que outras vacinas, em diferentes extremidades.
- A resposta imunológica pode diminuir no caso de tratamentos imunossupressores, como os corticosteroides, os medicamentos citotóxicos ou a radioterapia.
Gravidez elactação
Se está grávida ou em período de lactação, acredita que possa estar grávida, consulte o seu médico ou farmacêutico antes de utilizar esta vacina.
Vaxigrip Tetra pode ser utilizado em todas as fases da gravidez.
Vaxigrip Tetra pode ser usado durante o período de lactação.
O seu médico/farmacêutico poderá decidir se você deve ser vacinado com Vaxigrip Tetra.
Condução e uso de máquinas
Vaxigrip Tetra tem uma influência nula ou insignificante sobre a capacidade de conduzir e usar máquinas.
Vaxigrip Tetra contém potássio e sódio
Este medicamento contém menos de 1 mmol de potássio (39 mg) e menos de 1 mmol de sódio (23 mg) por dose; isto é, é essencialmente "isento de potássio" e "isento de sódio".
3. Como usar Vaxigrip Tetra
Posologia
Adultos: 1 dose de 0,5 ml.
Uso em crianças
As crianças desde os 6 meses até os 17 anos de idade recebem uma dose de 0,5 ml.
Se o seu filho tem menos de 9 anos e não foi previamente vacinado contra a gripe, deve ser administrada uma segunda dose de 0,5 ml após um intervalo de, pelo menos, 4 semanas.
Se está grávida, uma dose de 0,5 ml administrada durante a gravidez pode proteger o seu bebê desde o nascimento até os 6 meses de idade. Para mais informações, pergunte ao seu médico ou farmacêutico.
Como é administrado Vaxigrip Tetra
O seu médico ou enfermeiro administrará a dose recomendada da vacina com uma injeção no músculo ou sob a pele.
Se você ou seu filho receberem mais Vaxigrip Tetra do que devem
Em alguns casos, foi administrada involuntariamente mais doses do que a recomendada.
Nesses casos, quando foram notificados eventos adversos, estes estavam em linha com os descritos após a administração da dose recomendada (ver Seção 4).
Se você tiver alguma dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Posíveis efeitos adversos
Como todos os medicamentos, esta vacina pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Reações alérgicas
Entre em contato imediatamente com o seu médico ou profissional de saúde ou dirija-se imediatamente ao serviço de urgência do hospital mais próximo se você ou seu filho experimentarem reações alérgicas (notificadas como raras: podem afetar até 1 de cada 1.000 pessoas) que podem ser ameaçadoras para a vida. Os sintomas podem incluir erupção cutânea, coceira, urticária, vermelhidão, dificuldade para respirar, falta de ar, inchaço do rosto, lábios, garganta ou língua, pele fria e pegajosa, palpitações, tontura, fraqueza ou desfalecimento.
Outros efeitos adversos notificados em adultos e pessoas idosas
Muito frequentes (podem afetar mais de 1 de cada 10 pessoas)
- Dor de cabeça, dor muscular (mialgia), mal-estar geral (mal-estar) (1), dor no local da injeção.
(1) Frequente em pessoas idosas
Frequentes (podem afetar até 1 de cada 10 pessoas)
- Febre (2), calafrios, reações no local da injeção: vermelhidão (eritema), inchaço, endurecimento (induração).
(2) Pouco frequente em pessoas idosas
Pouco frequentes (podem afetar até 1 de cada 100 pessoas)
- Tontura (3), diarreia, sensação de doença (náuseas) (4), fadiga, reações no local da injeção: hematomas (equimose), coceira (prurito) e calor.
(3) Raro em adultos (4) Raro em pessoas idosas
- Sofocos: apenas observados em pessoas idosas.
- Inchaço dos gânglios do pescoço, axila ou virilha (linfadenopatia): apenas observado em adultos.
Raros (podem afetar até 1 de cada 1.000 pessoas)
- Anomalias na percepção do toque, dor, calor e frio (parestesia), sonolência, aumento da sudorese (hiperhidrose), cansaço incomum e fraqueza (astenia), doença de tipo gripal.
- Dor nas articulações (artralgia), desconforto no local da injeção: apenas observados em adultos.
Outros efeitos adversos notificados em crianças de 3 a 17 anos de idade
Muito frequentes (podem afetar mais de 1 de cada 10 pessoas)
- Dor de cabeça, dor muscular (mialgia), mal-estar geral (mal-estar), calafrios (5), reações no local da injeção: dor, inchaço, vermelhidão (eritema) (5), endurecimento (induração) (5).
(5) Frequente em crianças de 9 a 17 anos
Frequentes (podem afetar até 1 de cada 10 pessoas)
- Febre, hematoma no local da injeção (equimose).
Pouco frequentes (podem afetar até 1 de cada 100 pessoas) em crianças de 3 a 8 anos de idade:
- Diminuição temporária no número de certas células do sangue chamadas plaquetas; quando o número é baixo, pode dar origem à formação excessiva de hematomas na pele ou sangramento (trombocitopenia transitoria): apenas observado em uma criança de 3 anos de idade
- Queixumes, inquietude
- Tontura, diarreia, vômitos, dor abdominal superior, dor nas articulações (artralgia), fadiga, calor no local da injeção.
Pouco frequentes (podem afetar 1 de cada 100 pessoas) em crianças de 9 a 17 anos de idade:
- Diarreia, coceira no local da injeção (prurito).
Outros efeitos adversos notificados em crianças de 6 a 35 meses de idade
Muito frequentes (podem afetar mais de 1 de cada 10 pessoas):
- Vômitos (1), dor muscular (mialgia) (2), irritabilidade (3), perda de apetite (3), mal-estar geral (mal-estar) (2), febre.
(1) Pouco frequente em crianças de 24 a 35 meses de idade (2) Raro em crianças menores de 24 meses de idade
(3) Raro em crianças de 24 a 35 meses de idade.
- Reações no local da injeção: dor/dor à palpação, vermelhidão (eritema).
- Dor de cabeça: apenas observado em crianças a partir dos 24 meses de idade.
- Sonolência, choro anormal: apenas observados em crianças menores de 24 meses de idade.
Frequentes (podem afetar até 1 de cada 10 pessoas):
- Calafrios: apenas observado em crianças a partir dos 24 meses de idade em diante.
- Reações no local da injeção: endurecimento (induração), inchaço, hematoma (equimose)
Pouco frequentes (podem afetar até 1 de cada 100 pessoas):
- Diarreia, hipersensibilidade.
Raros (podem afetar até 1 de cada 1.000 pessoas):
- Doença de tipo gripal, reações no local da injeção: erupção, prurito (coceira).
Em crianças de 6 meses a 8 anos de idade que recebem 2 doses, os efeitos adversos são semelhantes tanto após a primeira dose quanto após a segunda dose. Alguns efeitos adversos podem ocorrer após a administração da segunda dose em crianças de 6 meses a 35 meses de idade.
Esses efeitos adversos, quando observados, normalmente ocorreram durante os 3 primeiros dias seguintes à vacinação e desapareceram por si mesmos entre 1 e 3 dias após o início. A maioria desses efeitos adversos foi de intensidade leve.
Em geral, os efeitos adversos foram menos frequentes em pessoas idosas do que em adultos e crianças.
Os seguintes efeitos adversos foram observados após a administração de Vaxigrip. Esses efeitos adversos também podem ocorrer com Vaxigrip Tetra:
- Dor situada na rota do nervo (neuralgia), ataques (convulsões), distúrbios neurológicos que podem resultar em rigidez no pescoço, confusão, entorpecimento, dor e fraqueza dos membros, perda do equilíbrio, perda de reflexos, paralisia de uma parte ou de todo o corpo (encefalomielite, neurite, síndrome de Guillain-Barré).
- Inflamação dos vasos sanguíneos (vasculite) que podem dar origem a erupções cutâneas e, em casos muito raros, a problemas passageiros de rim.
- Trombocitopenia transitoria, linfadenopatia, parestesia em outros grupos de idade distintos dos descritos anteriormente para esses efeitos secundários.
Comunicação de efeitos adversos
Se você ou seu filho experimentar qualquer tipo de efeito adverso, consulte o seu médico, farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste folheto informativo. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Vaxigrip Tetra
Mantenha este medicamento fora da vista e do alcance das crianças.
Não utilize esta vacina após a data de validade que aparece no envase após CAD. A data de validade é o último dia do mês que se indica.
Conservar na geladeira (2°C - 8°C). Não congelar. Conservar a seringa no embalagem exterior para protegê-la da luz.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os envases e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos envases e dos medicamentos que não precisa. Dessa forma, você ajudará a proteger o meio ambiente.
6. Conteúdo do envase e informação adicional
Composição de Vaxigrip Tetra
- Os princípios ativos são: Vírus da gripe (fracionados, inativados) das seguintes cepas*:
- Cepa semelhante a A/Victoria/4897/2022 (H1N1)pdm09: (IVR-238)........15 microgramas HA**
- Cepa semelhante a A/Croácia /10136RV/2023 (H3N2): (X-425)..............15 microgramas HA**
- Cepa semelhante a B/Áustria/1359417/2021: B/Michigan/01/2021 ..…....15 microgramas HA**
- B/Phuket/3073/2013..............................................................................15 microgramas HA**
Por dose de 0,5 ml
- cultivados em ovos de galinha embrionados procedentes de galinhas saudáveis
** hemaglutinina
Esta vacina cumpre com as recomendações da Organização Mundial da Saúde (Hemisfério Norte) e a decisão da União Europeia para a campanha 2025-2026.
- Os demais componentes são uma solução tampão que contém cloreto de sódio, fosfato de disódio dihidrato, fosfato dihidrogênio de potássio, cloreto de potássio e água para preparações injetáveis.
Alguns componentes, tais como ovos (ovoalbúmina, proteínas de galinha), neomicina, formaldeído ou octoxinol-9, podem estar presentes em quantidades muito pequenas (ver seção 2).
Aspecto do produto e conteúdo do envase
Depois de agitada cuidadosamente, a vacina é um líquido ligeiramente esbranquiçado e opalescente.
Vaxigrip Tetra é apresentado em uma seringa pré-carregada que contém 0,5 ml de suspensão injetável, com agulha fixa ou sem agulha (envases de 1, 10 ou 20) ou com agulha de segurança (em envases de 1 ou 10).
Pode ser que apenas alguns tamanhos de envases sejam comercializados.
Título da autorização de comercialização e responsável pela fabricação
Título da autorização de comercialização:
Sanofi Winthrop Industrie
82 avenue Raspail
94250 Gentilly
França
Responsável pela fabricação:
O fabricante responsável pela liberação dos lotes é:
Sanofi Winthrop Industrie
1541 avenue Marcel Mérieux
69280 Marcy l’Etoile
França
ou
Sanofi Winthrop Industrie
Voie de l’Institut – Parc Industriel d’Incarville
B.P 101
27100 Val de Reuil
França
ou
SANOFI-AVENTIS ZRT
Campona U.1 (Harbor Park )
Budapeste 1225
Hungria
Representante local
sanofi-aventis, S.A.
C/ Rosselló i Porcel, 21
08016 Barcelona
Espanha
Tel: +34 93 485 94 00
Este medicamento está autorizado nos estados membros do Espaço Económico Europeu com os seguintes nomes:
Estado Membro | Nome |
| VaxigripTetra Injektionssuspension in einer Fertigspritze VaxigripTetra injekcine suspensija užpildytame švirkšte VaxigripTetra Vaxigriptetra Vaxigrip Tetra suspension injectable en seringue préremplie Vaxigrip Tetra Quadrivalent influenza vaccine (split virion, inactivated) |
Data da última revisão desteprospecto: Maio 2025
Outras fontes de informação
Pode acessar informações detalhadas e atualizadas sobre este medicamento escaneando com seu telefone móvel (smartphone) o código QR incluído no cartonagem. Também pode acessar esta informação no seguinte endereço de internet: https://vaxigriptetra-nh.info.sanofi
--------------------------------------------------------------------------------------------------------------------------
Esta informação está destinada unicamente a médicos ou profissionais do setor sanitário:
Como com todas as vacinas injetáveis, deve-se dispor do tratamento médico e da supervisão apropriada no caso de que ocorra um episódio anafilático após a administração da vacina.
A vacina deve alcançar a temperatura ambiente antes de sua utilização.
Agite antes de usar.
A vacina não deve ser utilizada se apresentar partículas estranhas na suspensão.
Não se deve misturar com outros medicamentos na mesma seringa.
Esta vacina não deve ser injetada diretamente em nenhum vaso sanguíneo.
Ver também seção 3. Como usar Vaxigrip Tetra.
Preparação para a administração
Instruções de uso da agulha de segurança com a seringa pré-carregada Luer Lock:
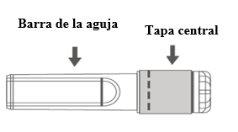
Imagem A: Agulha de segurança (dentro da barra) | Imagem B: Componentes da agulha de segurança (preparada para uso) |
|
|
Paso 1:Para fixar a agulha à seringa, retire a tampa central para expor a barra da agulha, e gire suavemente a agulha no adaptador Luer Lock da seringa até que note uma ligeira resistência. |
Paso 2:Extraia o protetor da agulha de segurança. A agulha está coberta pelo dispositivo de segurança e o protetor. |
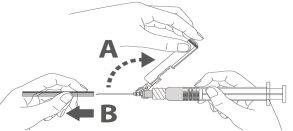
Paso 3: A:Separe o dispositivo de segurança da agulha em direção ao corpo da seringa no ângulo que se mostra. B:Retire o protetor em linha reta. |
|
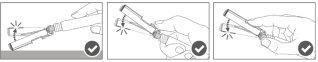
Paso 4:Uma vez finalizada a injeção, bloqueie (ative) o dispositivo de segurança utilizando uma das três técnicas ilustradas (3) com uma única mão: ativação com uma superfície, com o polegar ou com o dedo indicador. Nota: A ativação é verificada mediante um som "clic" e/ou táctil. |
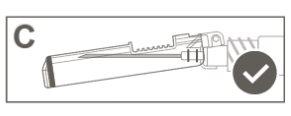
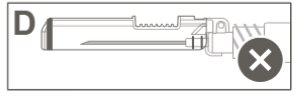
|
Paso 5:Inspeccione visualmente a ativação do dispositivo de segurança. O dispositivo de segurança deve estar completamente bloqueado (ativado)como se mostra na figura C. A figura D mostra que o dispositivo de segurança NÃO está completamente bloqueado (não ativado). |
|
Precaução: Não tente desbloquear (desativar) o dispositivo de segurança forçando a agulha para fora do dispositivo de segurança.> |
A eliminação das vacinas não utilizadas e de todos os materiais que tenham estado em contato com elas será realizada de acordo com a normativa local.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDAForma farmacêutica: INJETÁVEL, 3,75 microgramasSubstância ativa: influenza, inactivated, split virus or surface antigenFabricante: Glaxosmithkline BiologicalsRequer receita médicaForma farmacêutica: INJETÁVEL, 0,5 mlSubstância ativa: influenza, inactivated, split virus or surface antigenFabricante: Sanofi Winthrop IndustrieRequer receita médicaForma farmacêutica: INJETÁVEL, 60 microgramas de HASubstância ativa: influenza, inactivated, split virus or surface antigenFabricante: Sanofi Winthrop IndustrieRequer receita médica
Alternativas a VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA em Польша
Alternativa a VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA em Украина
Médicos online para VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de VAXIGRIP TETRA SUSPENSÃO INJETÁVEL EM SERINGA PREENCHIDA – sujeita a avaliação médica e regras locais.