TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO

Pergunte a um médico sobre a prescrição de TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO

Como usar TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO
Introdução
Prospecto: informação para o utilizador
TIMOFTOL 5 mg/ml colírio em solução
Timolol
Leia todo o prospecto detenidamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi-lhe prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é TIMOFTOL e para que é utilizado
- O que necessita de saber antes de começar a usar TIMOFTOL
- Como usar TIMOFTOL
- Posíveis efeitos adversos
- Conservação de TIMOFTOL
- Conteúdo do envase e informações adicionais
1. O que é TIMOFTOL e para que é utilizado
Timoftol é um agente betabloqueante oftálmico que pertence ao grupo de medicamentos denominado antiglaucomatosos tópicos.
Este medicamento está indicado para reduzir a pressão elevada no olho em:
- hipertensão ocular
- glaucoma crónico de ângulo aberto (incluindo pacientes afáquicos)
- algunos pacientes com glaucoma secundário.
2. O que necessita de saber antes de começar a usar TIMOFTOL
Não use TIMOFTOL
- Se é alérgico (hipersensível) ao timolol, aos betabloqueantes ou a qualquer um dos outros componentes de Timoftol (incluídos na secção 6).
- Se tem agora ou teve no passado certos problemas respiratórios importantes, tais como asma.
- Se tem agora ou teve no passado doença pulmonar obstructiva crónica (doença grave dos pulmões que pode ocasionar respiração sibilante, dificuldade respiratória e/ou tos persistente).
- Se tem certas doenças cardíacas (como latidos do coração lentos ou irregulares), bradicardia sinusal (frequência cardíaca inferior a 60 latidos por minuto), bloqueio aurículo-ventricular de segundo ou terceiro grau, insuficiência cardíaca manifesta ou choque cardiogénico.
- Se tem distrofia corneal (alteração degenerativa da córnea).
- Se tem rinite alérgica grave e hiperreatividade bronquial.
Advertências e precauções
Consulte o seu médico antes de começar a usar Timoftol.
Tenha especial cuidado com Timoftol.
Antes de utilizar este medicamento, informe o seu médico se tem ou teve no passado:
- problemas do coração, como doença coronária (os sintomas incluem dor torácica ou opressão, falta de ar ou ahogo), insuficiência cardíaca ou tensão arterial baixa
- alterações do ritmo do coração (como latidos do coração lentos ou irregulares)
- problemas da circulação (como a doença de Raynaud ou o síndrome de Raynaud)
- problemas respiratórios ou de pulmão (como asma ou doença pulmonar obstructiva crónica)
- diabetes ou outros problemas de açúcar no sangue, porque o timolol pode mascarar os sinais e sintomas de baixo nível de açúcar no sangue
- sobreactividade ou doença da tiróide, porque o timolol pode mascarar os sinais e sintomas
Diga ao seu médico, antes de uma intervenção cirúrgica, que está usando Timoftol, porque pode alterar os efeitos de alguns medicamentos usados durante a anestesia.
Também informe o seu médico sobre qualquer alergia ou medicação.
Timolol pode ser absorvido através do olho, chegando ao sangue, e podem produzir-se os mesmos efeitos adversos que com a administração de outros fármacos betabloqueantes orais.
- Se está tomando medicamentos betabloqueantes por via oral ou medicamentos antidepressivos inibidores da monoaminooxidase, deve informar o seu médico, porque podem aumentar os efeitos de Timoftol.
- Não se recomenda usar dois medicamentos betabloqueantes tópicos ao mesmo tempo.
- Se padece doença do seio, angina de Prinzmetal, feocromocitoma não tratado, acidose metabólica, distúrbios circulatórios periféricos graves (doença de Raynaud) ou tensão arterial baixa.
- Se utiliza lentes de contacto, não se recomenda o uso de Timoftol, porque aumenta o risco de apresentar intolerância às mesmas.
Como com qualquer tratamento para o glaucoma, é recomendável que o seu médico controle regularmente a pressão do olho e o estado da córnea.
Uso em desportistas
Este medicamento contém timolol, que pode produzir um resultado positivo nas provas de controlo de dopagem.
Crianças
Como regra geral, devem ser utilizados com precaução os colírios contendo timolol em solução nos pacientes jovens. No caso de recém-nascidos, lactentes e crianças de curta idade, o timolol deve ser utilizado com extrema precaução. Deve interromper-se imediatamente o uso deste medicamento no caso de aparecer tos, respiração sibilante, respiração anormal ou paragens anormais da respiração (apneia). Informe imediatamente o seu médico. Pode ser útil um controlador portátil de apneia.
Foi estudado o timolol em lactentes e crianças de idades compreendidas entre 12 dias e 5 anos, nos quais havia um aumento da pressão do (dos) olho(s) ou nos quais foi diagnosticado um glaucoma. Para mais informações, consulte o seu médico.
Uso de TIMOFTOL com outros medicamentos
Informe o seu médico ou farmacêutico se está utilizando, utilizou recentemente ou pode ter que utilizar qualquer outro medicamento, mesmo os adquiridos sem receita médica.
Certos medicamentos podem interagir com Timoftol, incluindo outros colírios para o tratamento do glaucoma, e nesses casos pode ser necessário alterar a dose ou interromper o tratamento com qualquer um deles. É importante que informe o seu médico se toma algum dos seguintes medicamentos:
- Betabloqueantes por via oral ou medicamentos que diminuam a tensão arterial, porque podem aumentar os efeitos de timolol sobre a pressão intraocular.
- Clonidina, porque pode produzir-se tensão arterial alta de rebote ao interromper o tratamento com clonidina.
- Medicamentos para o tratamento do ritmo do coração, como disopiramida, quinidina (utilizada também para tratar alguns tipos de malária) e amiodarona, porque timolol pode aumentar os seus efeitos.
- Medicamentos para a diabetes (insulina e antidiabéticos orais), porque timolol pode mascarar determinados sinais de hipoglicemia (açúcar no sangue baixo) como taquicardia (ritmo rápido do coração).
- Anestésicos.
- Medicamentos para a úlcera de estômago, como cimetidina.
- Álcool.
- Epinefrina, porque, juntamente com timolol, pode produzir dilatação da pupila (midriase).
- Medicamentos para a depressão, como fluoxetina ou paroxetina.
Gravidez e lactação
Se está grávida ou em período de lactação, acredita que possa estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Não utilize Timoftol se está grávida, a menos que o seu médico considere que seja necessário. Devido à possível ocorrência de efeitos adversos no feto, o seu médico avaliará o benefício/risco da administração de Timoftol.
Não utilize Timoftol se está em período de lactação. Timolol pode passar para o leite materno. Devido à possível ocorrência de efeitos adversos, o seu médico decidirá se suspender o tratamento com Timoftol ou suspender a lactação.
Condução e uso de máquinas
Timoftol pode fazer com que sinta tontura, fadiga ou visão borrosa, que podem afetar a sua capacidade para conduzir e utilizar máquinas.
TIMOFTOL contém cloreto de benzalconio e fosfatos
Este medicamento contém 0,11 mg de cloreto de benzalconio em cada ml.
O cloreto de benzalconio pode ser absorvido pelas lentes de contacto macias e pode alterar a cor das lentes de contacto. Retire as lentes de contacto antes de usar este medicamento e espere 15 minutos antes de voltar a colocá-las.
O cloreto de benzalconio pode causar irritação ocular, especialmente se padece de olho seco ou outras doenças da córnea (camada transparente da zona frontal do olho). Consulte o seu médico se sentir uma sensação estranha, coceira ou dor no olho após usar este medicamento.
Este medicamento contém 30,42 mg de hidrogenofosfato de disódio dodecahidrato e 6,10 mg de dihidrogenofosfato de sódio dihidrato em cada ml. Se sofre de dano grave na camada transparente da parte frontal do olho (córnea), o tratamento com fosfatos, em casos muito raros, pode provocar manchas nubladas na córnea devido ao cálcio.
3. Como usar TIMOFTOL
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Lembre-se de usar o medicamento.
O seu médico estabelecerá a dose e a duração do tratamento com Timoftol. Não suspenda o tratamento antes de tempo, porque cessaria o seu efeito benéfico.
Timoftol é um colírio para administração por via oftálmica.
A dose normal é uma gota de Timoftol 2,5 mg/ml no olho ou olhos afetados duas vezes ao dia. Se a resposta não for satisfatória, o seu médico pode aumentar a dose para uma gota de Timoftol 5 mg/ml no olho ou olhos afetados duas vezes ao dia.
O seu médico avaliará periodicamente a resposta ao tratamento com Timoftol e decidirá se é necessário complementá-lo com outros medicamentos disponíveis para reduzir a pressão intraocular.
Se está utilizando ao mesmo tempo outros colírios, deve esperar pelo menos 10 minutos entre as aplicações para que os princípios ativos não sejam eliminados do olho.
No caso de este colírio substituir outro tratamento para o glaucoma anterior ou ser empregado juntamente com outros medicamentos, o seu médico indicará a pauta que deve seguir.
Se estima que a ação de Timoftol é demasiado forte ou débil, comunique ao seu médico ou farmacêutico.
Uso em crianças e adolescentes
Antes da utilização de timolol, deve ser realizado um exame médico completo. O seu médico avaliará cuidadosamente os benefícios face aos riscos antes de planear um tratamento com timolol. Se os benefícios forem superiores aos riscos, recomenda-se utilizar uma vez ao dia a concentração mais baixa disponível de substância ativa.
Se não se controla suficientemente a pressão com esta concentração, pode ser necessária a administração duas vezes ao dia com 12 horas de intervalo entre elas. Deve controlar-se estreitamente os pacientes, especialmente os recém-nascidos, durante uma a duas horas posteriores à primeira administração, vigilando com atenção a ocorrência de efeitos adversos até que se leve a cabo a cirurgia. No caso do uso em crianças, para controlar a pressão no interior do olho, pode ser suficiente a concentração de 1 mg/ml em substância ativa, caso esteja disponível.
Duração do tratamento
Na população pediátrica, prescrever-se-á como um tratamento temporário.
Forma de administração
Em cada administração, apenas se deve instilar uma gota de Timoftol.
Após a instilação, manter os olhos fechados tanto tempo quanto for possível (por exemplo, 3 a 5 minutos) e manter pressionado com um dedo o ângulo do olho mais próximo do nariz, a fim de impedir a difusão da gota de timolol para o corpo.
Instruções de uso
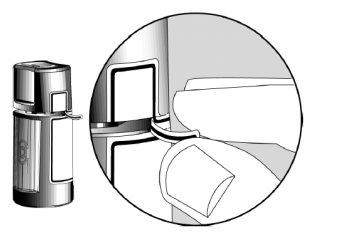
Não utilize o envase se a tira de segurança de plástico ao redor do pescoço do envase não estiver ou estiver quebrada. Quando abrir o envase pela primeira vez, arranque a tira de segurança de plástico.
[para envases distintos de OCUMETER PLUS:]
Cada vez que utilizar Timoftol:
| |
| |
|
|
|
|
|
|
[somente para envases OCUMETER PLUS:]
- Antes de utilizar o medicamento pela primeira vez, certifique-se de que a Tira de Segurança na parte da frente do frasco está intacta. Quando o frasco não foi aberto ainda, é normal a existência de um espaço entre o frasco e o capuchão.
Setas de Abertura ?
Tira de segurança ?
- Lave as mãos. Quando abrir o frasco pela primeira vez, arranque a Tira de Segurança para romper o precinto.
Espaço ?
Área para Pressionar com o
Dedo ?
- Para abrir o frasco, desenrosque o capuchão girando-o segundo as indicações das setas da parte de cima do capuchão. Não puxe diretamente o capuchão do frasco para cima. Puxar o capuchão para cima impedirá que o dispensador funcione corretamente.

Área para Pressionar com o
Dedo ?
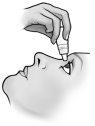
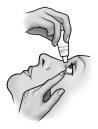
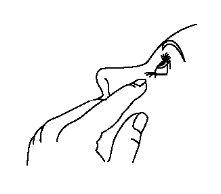
- Incline a cabeça para trás e separe o párpado inferior ligeiramente, formando uma pequena separação entre o párpado e o olho.
- Inverta o frasco e pressione ligeiramente com o dedo polegar ou com o índice sobre o “Área para Pressionar Com o Dedo” (como mostra a figura seguinte) até dispensar uma única gota no olho, de acordo com as instruções do seu médico.
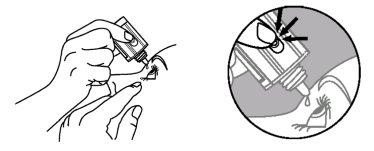
Área para Pressionar
com o Dedo
NÃO TOQUE O OLHO NEM O PÁRPADO COM A PONTA DO TAMPÃO CONTA-GOTAS.
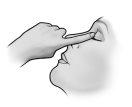
- Após usar Timoftol, pressione com o dedo na esquina do olho ao lado do nariz (como mostra a figura seguinte) durante 2 minutos. Isso ajuda a manter Timoftol no olho e impede a difusão de timolol para o resto do corpo.
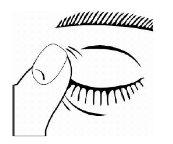
- Se, após abrir pela primeira vez, a dispensação da gota for difícil, coloque novamente o capuchão e aperte (NÃO APERTe DEMAIS) e, em seguida, retire o capuchão girando-o na direção contrária, como está indicado nas setas da parte de cima do capuchão.
- Repita os passos 4 e 5 no outro olho, se assim o indicar o seu médico.
- Feche o capuchão girando-o até que toque a borda do frasco. Para um fechamento adequado, a seta do lado esquerdo do capuchão deve estar alinhada com a seta do lado esquerdo da etiqueta do frasco. Não o aperte demais ou pode estragar o frasco e o capuchão.
- A ponta do dispensador está projetada para fornecer uma única gota; portanto, NÃO alargue o orifício da ponta do dispensador.
- Após ter usado todas as doses, restará um pouco de Timoftol no frasco. Não deve se preocupar, pois foi adicionada uma quantidade extra de Timoftol e você obterá a quantidade total de Timoftol que o seu médico prescreveu. Não tente extrair o excesso de medicamento do frasco.
Os medicamentos oftálmicos, se utilizados inadequadamente, podem ser contaminados com bactérias comuns conhecidas por causar infecções nos olhos. O uso de soluções oftálmicas contaminadas pode dar origem a distúrbios oculares graves e à subsequente perda da visão. Se achar que o seu medicamento pode estar contaminado, ou se desenvolver uma infecção no olho, entre em contato com o seu médico imediatamente sobre o uso contínuo desse frasco.
Se usar mais TIMOFTOL do que deve
Se utilizou mais Timoftol do que deve, consulte imediatamente o seu médico ou farmacêutico.
Os sintomas mais comuns em caso de sobredose com timolol são: tontura, cefaleia, respiração entrecortada, diminuição do número de batimentos cardíacos, descenso da tensão arterial, insuficiência cardíaca e/ou paro cardíaco.
Em caso de sobredose ou ingestão acidental, consulte imediatamente o seu médico ou farmacêutico ou ligue para o Serviço de Informação Toxicológica, telefone 91.562.04.20, indicando o medicamento e a quantidade utilizada.
Se esquecer de usar TIMOFTOL
Não use uma dose dupla para compensar as doses esquecidas.
Use Timoftol segundo a pauta que o seu médico indicou. Se esquecer uma dose, administre-a o mais breve possível. No entanto, se já é quase a hora da próxima dose, ignore a dose esquecida e volte à sua pauta de administração habitual.
4. Possíveis efeitos adversos
Tal como todos os medicamentos, o Timoftol pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Normalmente poderá continuar a utilizar as gotas, a menos que os efeitos sejam graves. Se está preocupado, consulte o seu médico ou farmacêutico. Não interrompa o uso do Timoftol sem comentar antes com o seu médico.
Com a administração de timolol por via oftálmica, foram observados os seguintes efeitos adversos:
Frequentes (podem afetar até 1 de cada 10 doentes):
- dor de cabeça
- signos e sintomas de irritação ocular (p. ex., sensação de queimadura, picadas, coceira, conjuntivite, lacrimejamento, vermelhidão), inflamação das pálpebras, inflamação da córnea, diminuição da sensibilidade corneal e olhos secos
Pouco frequentes (podem afetar até 1 de cada 100 doentes):
- depressão
- tonturas, desmaio
- distúrbios visuais como alterações refractivas
- ritmo cardíaco lento (bradicardia)
- dificuldade na respiração (dispneia)
- náuseas, digestão pesada (dispepsia)
- fadiga/cansancio
Raros (podem afetar até 1 de cada 1.000 doentes):
- signos e sintomas de reações alérgicas, incluindo angioedema (inchaço debaixo da pele em zonas como a face e as extremidades, e que pode obstruir as vias respiratórias e pode causar dificuldade ao engolir ou respirar), urticária ou erupção cutânea com coceira, erupção localizada e generalizada, anafilaxia (reação alérgica grave que pode pôr em perigo a vida)
- insónia (dificuldade para dormir), pesadelos, perda de memória
- sensação de formigamento ou agulhetas, aumento nos signos e sintomas de miastenia gravis (distúrbio muscular), diminuição do apetite sexual (libido diminuída), acidente vascular cerebral (AVC), isquemia cerebral (chegada reduzida de sangue ao cérebro)
- pálpebra superior caída (ficando o olho meio fechado), visão dupla (diplopia), desprendimento coroide (desprendimento da camada vascular debaixo da retina após a cirurgia de filtração que pode causar alterações na visão)
- ruídos nos ouvidos (acúfenos)
- dor torácica, palpitações, inchaço, ritmo cardíaco irregular, insuficiência cardíaca congestiva (doença caracterizada por dificuldade para respirar e inchaço de pés e pernas devido à acumulação de líquidos), bloqueio cardíaco, parada cardíaca
- pressão arterial baixa, fenómeno de Raynaud (distúrbio dos vasos sanguíneos que afeta geralmente os dedos das mãos e pés), dor ou desconforto em uma extremidade ao começar a caminhar, mãos e pés frios
- problemas para respirar e constrição das vias respiratórias (predominante em doentes com doença broncoespástica pré-existente), tosse
- diarreia, boca seca
- perda de cabelo, erupção cutânea psoriasiforme ou exacerbação de psoríase
- doença inflamatória com febre, fraqueza, dor nas articulações e lesões na pele (lúpus eritematoso sistémico)
- doença de Peyronie (que pode causar uma marcada curvatura do pênis)
Frequência desconhecida (não pode ser estimada a partir dos dados disponíveis):
- alucinações
Como os outros medicamentos que se aplicam nos olhos, o timolol passa para o sangue. Isso pode ocasionar efeitos adversos semelhantes aos observados com os agentes betabloqueantes administrados por via oral ou injetáveis. A ocorrência de efeitos adversos por via tópica oftálmica é menos frequente do que no caso de administração por via oral ou injetável. Os efeitos adversos listados incluem aqueles que se têm observado na classe dos betabloqueantes utilizados para tratar as doenças oculares:
- Reações alérgicas generalizadas, incluindo inchaço debaixo da pele em zonas como a face e as extremidades, e que pode obstruir as vias respiratórias e pode causar dificuldade ao engolir ou respirar, urticária ou erupção cutânea com coceira, erupção localizada e generalizada, picote, reação alérgica grave que pode pôr em perigo a vida.
- Nível baixo de glicose no sangue.
- Dificuldade para dormir (insónia), depressão, pesadelos, perda de memória.
- Desmaio, acidente vascular cerebral (AVC), redução do aporte sanguíneo ao cérebro, piora dos signos e sintomas de miastenia gravis (distúrbio muscular), tonturas, sensações estranhas como agulhetas e dor de cabeça.
- Signos e sintomas de irritação ocular (p. ex., queimadura, picadas, coceira, lacrimejamento, vermelhidão), inflamação da pálpebra, inflamação da córnea, visão borrada e desprendimento da camada vascular debaixo da retina após a cirurgia de filtração que pode causar alterações na visão, diminuição da sensibilidade corneal, olhos secos, erosão corneal (dano na camada frontal do globo ocular), pálpebra superior caída (ficando o olho meio fechado), visão dupla.
- Ritmo cardíaco lento, palpitações, dor torácica, edema (aumento de líquido), alterações na velocidade ou ritmo do batimento cardíaco, insuficiência cardíaca congestiva (doença caracterizada por dificuldade para respirar e inchaço de pés e pernas devido à acumulação de líquidos), algum distúrbio no ritmo cardíaco, bloqueio cardíaco, parada cardíaca
- Pressão arterial baixa, fenómeno de Raynaud, mãos e pés frios.
- Constrição das vias respiratórias (predominante em doentes com doença pré-existente), dificuldade para respirar, tosse.
- Alteração do gosto, náuseas, indigestão, diarreia, boca seca, dor abdominal, vômitos.
- Perda de cabelo, erupção cutânea de cor branco-prateado (erupção psoriasiforme) ou piora da psoríase, erupção cutânea.
- Dor muscular não causada por exercício.
- Disfunção sexual, diminuição da libido.
- Fraqueza muscular/cansancio.
Comunicação de efeitos adversos:
Se experimentar qualquer tipo de efeito adverso, consulte o seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste folheto. Também pode comunicá-los diretamente através do Sistema Espanhol de Farmacovigilância de medicamentos de Uso Humano: https://www.notificaram.es.
Ao comunicar efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação do TIMOFTOL
Mantenha este medicamento fora da vista e do alcance das crianças.
Não conserve a uma temperatura superior a 25 ºC.
Conservar no embalagem original para protegê-lo da luz.
Não utilize este medicamento após a data de validade que aparece no invólucro após a abreviatura CAD.
A data de validade é o último dia do mês que se indica.
Elimine quatro semanas após a abertura do invólucro.
Os medicamentos não devem ser jogados nos esgotos nem na lixeira. Deposite os invólucros e os medicamentos que não precisa no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos invólucros e dos medicamentos que não precisa. Dessa forma, você ajudará a proteger o meio ambiente.
6. Conteúdo do invólucro e informações adicionais
Composição do TIMOFTOL
- O princípio ativo é timolol. Cada ml de Timoftol contém 6,8 mg de maleato de timolol, equivalentes a 5 mg de timolol
- Os demais componentes são dihidrogenofosfato de sódio dihidrato, hidrogenofosfato de disódio dodecahidrato, hidróxido de sódio, cloruro de benzalconio e água para preparações injetáveis.
Aspecto do produto e conteúdo do invólucro
O Timoftol é apresentado sob a forma de colírio em solução transparente, incolor ou amarelo claro.
É apresentado em dois invólucros alternativos:
- Invólucro com conta-gotas que contém 5 ml de solução.
- Dispensador oftálmico Ocumeter Plus que contém 3 ml de solução.
Pode ser que apenas alguns tamanhos de invólucros estejam comercializados.
Titular da autorização de comercialização e responsável pela fabricação
Titular da autorização de comercialização
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finlândia
Responsável pela fabricação
Laboratoires Merck Sharp & Dohme
Chibret (“MIRABEL PLANT”)
Route de Marsat, RIOM
63963 Clermont – Ferrand, Cedex 9, França
Ó
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finlândia
Representante local
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
Espanha
Data da última revisão deste folheto: Abril 2020
A informação detalhada e atualizada deste medicamento está disponível na página Web da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/.
- País de registo
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃOForma farmacêutica: COLÍRIO, 5 mg/mlSubstância ativa: timololFabricante: Immedica Pharma AbRequer receita médicaForma farmacêutica: GEL OFTÁLMICO, 1 mg/gSubstância ativa: timololFabricante: Sifi S.P.A.Requer receita médicaForma farmacêutica: COLÍRIO, 0,25%Substância ativa: timololFabricante: Laboratorios Thea S.A.Requer receita médica
Alternativas a TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO em Polónia
Alternativa a TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO em Ukraine
Médicos online para TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de TIMOFTOL 5 mg/ml COLÍRIO EM SOLUÇÃO – sujeita a avaliação médica e regras locais.