BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico
Pergunte a um médico sobre a prescrição de BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico

Como usar BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico
Introdução
Prospecto: informação para o utilizador
Buprenorfina Teva 35 microgramas/hora adesivo transdérmico EFG
Buprenorfina
Leia todo o prospecto atentamente antes de começar a usar este medicamento, porque contém informações importantes para si.
- Conserva este prospecto, porque pode ter que voltar a lê-lo.
- Se tiver alguma dúvida, consulte o seu médico ou farmacêutico.
- Este medicamento foi prescrito apenas para si, e não deve dá-lo a outras pessoas, mesmo que tenham os mesmos sintomas que si, porque pode prejudicá-las.
- Se experimentar efeitos adversos, consulte o seu médico ou farmacêutico, mesmo que se trate de efeitos adversos que não aparecem neste prospecto. Ver secção 4.
Conteúdo do prospecto
- O que é Buprenorfina Teva e para que é utilizado
- O que precisa saber antes de começar a usar Buprenorfina Teva
- Como usar Buprenorfina Teva
- Posíveis efeitos adversos
- Conservação de Buprenorfina Teva
- Conteúdo do envase e informações adicionais
1. O que é Buprenorfina Teva e para que é utilizado
A substância ativa de Buprenorfina Teva é buprenorfina.
Buprenorfina Teva é um analgésico (um medicamento para aliviar a dor) indicado para aliviar a dor moderada a severa oncológica e a dor severa que não responde a outros tipos de analgésicos. Buprenorfina Teva actua através da pele. Quando se aplica o adesivo transdérmico sobre a pele, o princípio ativo buprenorfina passa através da pele para o sangue. A buprenorfina é um opioide (medicamento para aliviar a dor intensa) que reduz a dor actuando sobre o sistema nervoso central (em células nervosas específicas na medula espinhal e no cérebro). O efeito do adesivo transdérmico dura até um máximo de quatro dias. Buprenorfina Teva não é adequado para o tratamento da dor aguda (de curta duração).
2. O que precisa saber antes de começar a usar Buprenorfina Teva
Não use Buprenorfina Teva
- se é alérgico à buprenorfina ou a qualquer um dos outros componentes deste medicamento (incluídos na secção 6),
- se é viciado em analgésicos potentes (opioides),
- se padece uma doença em que tem grande dificuldade para respirar ou em que isso pode ocorrer-lhe,
- se está a tomar inibidores da MAO (certos medicamentos para o tratamento da depressão) ou os tomou nas duas últimas semanas antes do tratamento com Buprenorfina Teva (ver “Outros medicamentos e Buprenorfina Teva”),
- em caso de miastenia gravis (um tipo de fraqueza muscular severa),
- em caso de delirium tremens (confusão e tremor causados pela abstinência de álcool após uma ingestão excessiva habitual do mesmo ou durante um episódio de consumo elevado de álcool),
- em caso de gravidez.
Buprenorfina Teva não se deve utilizar para tratar a síndrome de abstinência em drogodependentes.
Advertências e precauções
Consulte o seu médico ou farmacêutico antes de começar a usar Buprenorfina Teva
- se bebeu muito álcool recentemente,
- se tem crises epilépticas ou convulsões (ataques),
- se tem a consciência alterada (sensação de tontura ou desmaio), por causa desconhecida,
- se está em estado de choque (um sinal pode ser o suor frio),
- se tem a pressão craniana elevada (por exemplo após traumatismo craneoencefálico ou em doença cerebral), sem a possibilidade de respiração artificial,
- se tem dificuldade para respirar ou está a tomar outra medicação que pode fazer-lhe respirar mais lentamente ou debilmente (ver “Outros medicamentos e Buprenorfina Teva”),
- se tem depressão ou outras doenças que se tratam com antidepressivos.
O uso destes medicamentos juntamente com Buprenorfina Teva pode provocar síndrome serotoninérgico, uma doença potencialmente mortal (ver «Outros medicamentos e Buprenorfina Teva»),
- se tem problemas hepáticos,
- se tem tendência para abusar de medicamentos ou drogas.
Tenha em conta também as seguintes precauções:
- Algumas pessoas podem chegar a depender de analgésicos potentes, tais como Buprenorfina Teva, quando os utilizam durante muito tempo. Estes pacientes podem ter efeitos após deixar de utilizar o medicamento (ver “Se interromper o tratamento com Buprenorfina Teva”).
- A febre e o calor ambiental podem dar origem a quantidades maiores do que as normais de buprenorfina no sangue. Além disso, o calor ambiental pode impedir que o adesivo transdérmico se pegue adequadamente. Por isso, consulte o seu médico se tiver febre e não se exponha a fontes de calor (ex.: sauna, lâmpadas infravermelhas, mantas elétricas ou bolsas de água quente).
- Distúrbios respiratórios relacionados com o sono: Buprenorfina Teva pode causar distúrbios respiratórios relacionados com o sono, tais como apneia de sono (pausas respiratórias durante o sono) e hipoxemia relacionada com o sono (níveis baixos de oxigênio no sangue). Os sintomas podem incluir pausas respiratórias durante o sono, despertares noturnos devido à dificuldade para respirar, dificuldade para manter o sono ou sonolência excessiva durante o dia. Contacte o seu médico se você ou outra pessoa observa estes sintomas. O seu médico poderia considerar uma redução da dose.
- Deve-se advertir os desportistas que este medicamento pode dar um resultado positivo nos testes de controlo do dopagem.
Tolerância, dependência e adicção
Este medicamento contém buprenorfina, uma substância opioide. O uso repetido de opioides pode diminuir a eficácia do medicamento (o seu organismo se acostuma ao medicamento, isto é o que se conhece como tolerância). O uso repetido de Buprenorfina Teva também pode causar dependência, abuso e adicção, o que pode dar origem a uma sobredose potencialmente mortal. O risco de efeitos adversos pode aumentar com uma dose mais alta e uma duração de uso mais prolongada. A dependência ou a adicção podem fazer-lhe sentir que já não tem o controlo da quantidade de medicamento que precisa tomar ou com que frequência deve tomá-lo.
O risco de se tornar dependente ou adicto varia de acordo com a pessoa. Pode apresentar um maior risco de se tornar dependente ou adicto a Buprenorfina Teva se:
- Si ou algum membro da sua família tem antecedentes de abuso ou dependência de álcool, medicamentos de venda com receita ou substâncias ilícitas (“adicção”).
- É fumador.
- Alguma vez teve problemas com o seu estado de ânimo (depressão, ansiedade ou um distúrbio de personalidade) ou recebeu tratamento de um psiquiatra para outras doenças mentais.
Se nota algum dos seguintes sinais enquanto toma Buprenorfina Teva, poderia ser um sinal de que se tornou dependente ou adicto:
- Precisa tomar o medicamento durante mais tempo do que o recomendado pelo seu médico.
- Precisa tomar mais doses do que a recomendada.
- É possível que sinta que precisa continuar a usar o seu medicamento, mesmo quando não o ajude a aliviar a dor.
- Está a usar o medicamento por razões distintas das prescritas, por exemplo, “para se acalmar” ou “para o ajudar a dormir”.
- Fez tentativas repetidas e sem sucesso de deixar ou controlar o uso do medicamento.
- Não se encontra bem quando deixa de tomar o medicamento e se sente melhor quando volta a tomá-lo (“sintomas de abstinência”).
Se nota algum destes sinais, fale com o seu médico para abordar a estratégia terapêutica mais adequada no seu caso, incluindo quando é apropriado deixar de tomar o medicamento e como fazê-lo de forma segura (ver secção 3 “Se interromper o tratamento com Buprenorfina Teva”).
Crianças e adolescentes
Buprenorfina Teva não se deve utilizar em pessoas menores de 18 anos, porque não se tem experiência até ao momento neste grupo de idade.
Outros medicamentos e Buprenorfina Teva
Comunique ao seu médico ou farmacêutico se está a utilizar, utilizou recentemente ou poderia ter que utilizar qualquer outro medicamento.
- Buprenorfina Teva não se deve utilizar juntamente com inibidores da MAO (certos medicamentos para o tratamento da depressão), ou se os tomou nas duas últimas semanas.
- Buprenorfina Teva pode produzir em alguns pacientes sonolência, vómitos, tonturas ou fazer-lhes respirar mais lentamente ou debilmente. Estes efeitos adversos podem intensificar-se se se tomam ao mesmo tempo outros medicamentos que podem produzir os mesmos efeitos. Estes outros medicamentos incluem outros analgésicos potentes (opioides), certos medicamentos para dormir, anestésicos e medicamentos para o tratamento de certas doenças psicológicas como tranquilizantes, antidepressivos e neurolépticos.
O uso concomitante de Buprenorfina Teva e medicamentos sedantes como benzodiazepinas ou medicamentos relacionados aumenta o risco de sonolência, dificuldade para respirar (depressão respiratória), coma e pode ser potencialmente mortal. Devido a isso, o uso concomitante só se deve considerar quando outras opções de tratamento não são possíveis.
No entanto, se o seu médico lhe prescreve Buprenorfina Teva juntamente com medicamentos sedantes, o seu médico deve limitar a dose e a duração do tratamento concomitante.
Informa ao seu médico sobre todos os medicamentos sedantes que está a tomar e siga de perto a recomendação da dose. Poderia ser útil informar amigos ou familiares para que estejam a par dos sinais e sintomas indicados anteriormente. Contacte o seu médico quando experimente tais sintomas.
O uso concomitante de Buprenorfina Teva e gabapentinoides como gabapentina ou pregabalina utilizados para tratar a epilepsia ou a dor devido a problemas nerviosos (dor neuropática) pode provocar dificuldades para respirar (depressão respiratória), pressão arterial baixa, sonolência profunda, coma e pode ser potencialmente mortal.
- Se Buprenorfina Teva se utilizar juntamente com anticolinérgicos ou medicamentos com atividade anticolinérgica, como medicamentos para tratar a depressão, medicamentos utilizados para tratar alergias, tonturas ou náuseas (antihistamínicos ou antieméticos), medicamentos para tratar distúrbios psiquiátricos (antipsicóticos ou neurolépticos), relaxantes musculares ou medicamentos para tratar a doença de Parkinson, os efeitos secundários anticolinérgicos podem aumentar.
- Se Buprenorfina Teva se utilizar juntamente com alguns medicamentos, a ação do adesivo transdérmico pode intensificar-se. Estes medicamentos incluem por exemplo certos anti-infecciosos e antifúngicos (ex.: aqueles que contêm eritromicina ou cetoconazol) ou medicamentos para o VIH (ex.: aqueles que contêm ritonavir).
- Se Buprenorfina Teva se utilizar juntamente com outros medicamentos, a ação do adesivo transdérmico pode reduzir-se. Estes medicamentos incluem por exemplo dexametasona, certos produtos para o tratamento da epilepsia (ex.: aqueles que contêm carbamazepina ou fenitoína) ou medicamentos que se utilizam para o tratamento da tuberculose (ex.: rifampicina).
- Alguns medicamentos podem aumentar os efeitos secundários de Buprenorfina Teva e, por vezes, podem provocar reações muito graves. Não tome qualquer outro medicamento enquanto estiver a tomar Buprenorfina Teva sem consultar primeiro o seu médico, especialmente antidepressivos como moclobemida, tranilcipromina, citalopram, escitalopram, fluoxetina, fluvoxamina, paroxetina, sertralina, duloxetina, venlafaxina, amitriptilina, doxepina ou trimipramina. Estes medicamentos podem interagir com Buprenorfina Teva e pode experimentar sintomas como contracções musculares rítmicas involuntárias, incluídos os músculos que controlam o movimento dos olhos, agitação, alucinações, coma, suoração excessiva, tremores, exageração dos reflexos, aumento da tensão muscular, temperatura corporal superior a 38 °C. Contacte o seu médico se sofrer estes sintomas.
Uso de Buprenorfina Teva com alimentos, bebidas e álcool
Não deve beber álcool enquanto utilizar Buprenorfina Teva. O álcool pode intensificar certos efeitos adversos do adesivo transdérmico e pode que não se encontre bem. Se você bebe sumo de toranja, pode intensificar os efeitos de Buprenorfina Teva.
Gravidez, lactação e fertilidade
Se está grávida ou em período de lactação, acredita que poderia estar grávida ou tem intenção de ficar grávida, consulte o seu médico ou farmacêutico antes de utilizar este medicamento.
Não existe experiência suficiente do uso de Buprenorfina Teva até ao momento em mulheres grávidas. Por isso, não utilize Buprenorfina Teva durante a gravidez.
Buprenorfina, o princípio ativo contido no adesivo transdérmico, inibe a produção de leite e passa para o leite materno. Por isso, não utilize Buprenorfina Teva durante a lactação.
Condução e uso de máquinas
Buprenorfina Teva pode fazer-lhe sentir tonturas, sonolência ou ter visão dupla ou borrosa e pode alterar os seus reflexos de forma que não reaja adequadamente ou suficientemente rápido em caso de situações súbitas ou inesperadas. Isto aplica-se especialmente:
- no início do tratamento
- quando mudar a dose
- quando mudar de outro medicamento para Buprenorfina Teva
- se você também tomar outros medicamentos que actuam no cérebro
- se você beber álcool
Se estiver afectado, não deve conduzir ou manejar máquinas enquanto utilizar Buprenorfina Teva. Isto também se aplica ao final do tratamento com Buprenorfina Teva. Não conduza ou maneje máquinas pelo menos durante as 24 horas posteriores à retirada do adesivo.
Em caso de dúvida, consulte o seu médico ou farmacêutico.
3. Como usar Buprenorfina Teva
Siga exatamente as instruções de administração deste medicamento indicadas pelo seu médico. Em caso de dúvida, consulte novamente o seu médico ou farmacêutico.
Antes de iniciar o tratamento e de forma periódica durante o mesmo, o seu médico falará com você sobre o que pode esperar do uso deste medicamento, quando e durante quanto tempo deve tomá-lo, quando entrar em contato com o seu médico e quando deve deixar de tomá-lo (ver também “Se interromper o tratamento com Buprenorfina Teva”).
Este medicamento está disponível em três doses: Buprenorfina Teva 35 microgramas/hora adesivo transdérmico EFG, Buprenorfina Teva 52,5 microgramas/hora adesivo transdérmico EFG e Buprenorfina Teva 70 microgramas/hora adesivo transdérmico EFG.
Seu médico escolheu este adesivo de buprenorfina como o mais adequado para você. Durante o tratamento, seu médico pode mudar o adesivo transdérmico que você usa por outro de dose menor ou maior, se necessário.
A dose recomendada é:
Adultos
Siga estas instruções a menos que seu médico tenha dado outras indicações distintas. Aplique o adesivo de buprenorfina (como detalhado abaixo) e mude-o após quatro dias, no máximo. Para facilitar seu uso, você pode mudar o adesivo 2 vezes por semana em dias fixos, por exemplo: “sempre às segundas-feiras de manhã e às quintas-feiras à tarde”. Para ajudá-lo a lembrar quando deve mudar o adesivo transdérmico, anote no calendário do cartucho. Se seu médico indicou que você tome outros analgésicos além do adesivo transdérmico, siga estritamente as instruções do seu médico; caso contrário, você não se beneficiará completamente do tratamento com buprenorfina.
Crianças e adolescentes
Este medicamento não deve ser usado em pessoas menores de 18 anos porque, até o momento, não há experiência nesse grupo etário.
Pacientes idosos
Não é necessário ajuste de dose em pacientes idosos.
Pacientes com alterações renais / pacientes submetidos a diálise
Em pacientes com alteração renal e pacientes submetidos a diálise, não é necessário ajuste de dose.
Pacientes com alteração hepática
Em pacientes com alteração hepática, a intensidade e duração da ação de buprenorfina podem ser afetadas. Se você pertence a este grupo de pacientes, seu médico o controlará com maior cuidado.
Método de administração
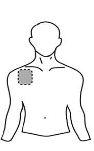
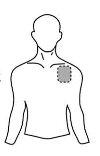
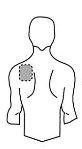
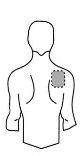
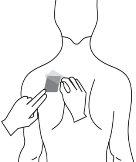
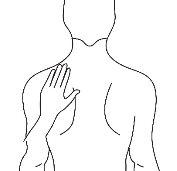
Antes de aplicar um adesivo transdérmico
| Peito |
|
|
Costas |
|
|
- Se a área escolhida tiver pelos, corte-os com tesouras. Não os afeite!
- Evite áreas da pele avermelhadas, irritadas ou com qualquer outro tipo de manchas, por exemplo, grandes cicatrizes.
- A área da pele que você escolher deve estar seca e limpa. Se necessário, lave-a com água fria ou morna. Não use sabão ou outros detergentes. Depois de um banho quente ou chuveiro, espere até que sua pele esteja completamente seca e fria. Não aplique loções, cremes ou pomadas na área escolhida. Isso poderia impedir que o adesivo transdérmico grude adequadamente.
Aplicação do adesivo transdérmico:
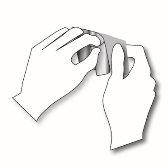
Passo 1: Cada adesivo transdérmico está selado em um envelope. Justo antes de usá-lo, corte o envelope ao longo da borda selada com tesouras. Tenha cuidado para não danificar os adesivos transdérmicos. Pegue o adesivo transdérmico. |
|
Passo 2: O lado adesivo do adesivo transdérmico está coberto por uma lâmina protetora transparente. Descole cuidadosamente a metadeda lâmina. Tente não tocar a parte adesiva do adesivo transdérmico. |
|
Passo 3: Cole o adesivo transdérmico na área da pele que você escolheu e retire o resto da lâmina. |
|
Passo 4: Pressione o adesivo transdérmico contra sua pele com a palma da sua mão e conte lentamente até 30. Certifique-se de que todo o adesivo transdérmico está em contato com sua pele, especialmente as bordas. |
|
Passo 5: Lave as mãos após usar o adesivo transdérmico. Não use nenhum produto de limpeza. | |
Enquanto usar o adesivo transdérmico
Você pode usar o adesivo transdérmico por no máximo 4 dias. Se você aplicou o adesivo transdérmico corretamente, o risco de que ele se descole é baixo. Você pode tomar banho, se banhar ou nadar enquanto o usa. No entanto, não exponha o adesivo transdérmico a calor extremo (por exemplo, sauna, lâmpadas infravermelhas, mantas elétricas ou bolsas de água quente).
No caso improvável de que seu adesivo transdérmico caia antes de que você precise mudá-lo, não use o mesmo adesivo transdérmico novamente. Cole um novo imediatamente (ver “Mudança do adesivo transdérmico” abaixo).
Mudança do adesivo transdérmico
- Remova com cuidado o adesivo velho.
- Dobre-o ao meio com o lado adesivo para dentro.
- Descarte-o com precaução, fora da vista e do alcance das crianças.
- Cole um novo adesivo transdérmico sobre uma área diferente da pele (como descrito anteriormente). Deve transcorrer pelo menos 1 semana antes de poder aplicar um novo adesivo na mesma área da pele.
Duração do tratamento
Seu médico indicará a duração do seu tratamento com este medicamento. Não suspenda o tratamento com buprenorfina por sua conta, pois a dor pode voltar a aparecer e você pode se sentir mal (ver também “Se interromper o tratamento com Buprenorfina Teva”).
Se você achar que o efeito deste medicamento é muito forte ou fraco, comunique ao seu médico ou farmacêutico.
Se usar mais Buprenorfina Teva do que deve
Se isso ocorrer, podem existir sinais de uma sobredosagem por buprenorfina. Uma sobredosagem pode intensificar os efeitos adversos de buprenorfina, tais como sonolência, náuseas e vômitos. Você pode ter as pupilas puntiformes e a respiração pode se tornar lenta e débil. Você também pode sofrer um colapso cardiovascular.
Assim que se der conta de que usou mais adesivos transdérmicos do que deve, remova os adesivos transdérmicos em excesso e consulte imediatamente seu médico ou farmacêutico.
Em caso de sobredosagem ou ingestão acidental, consulte o Serviço de Informação Toxicológica, telefone 91 562 04 20, indicando o medicamento e a quantidade usada
Se esquecer de usar Buprenorfina Teva
Se você esqueceu uma aplicação, cole um novo adesivo transdérmico assim que se der conta. Isso fará com que você mude sua rotina, por exemplo: se normalmente aplicava seu adesivo transdérmico às segundas-feiras e às quintas-feiras, mas esqueceu e não colocou o novo adesivo transdérmico até a quarta-feira, a partir de agora você precisará mudar seus adesivos transdérmicos às quartas-feiras e aos sábados. Anote o novo par de dias no calendário do cartucho. Se mudar o adesivo transdérmico muito tarde, a dor pode voltar a aparecer. Nesse caso, consulte seu médico.
Nunca se aplique mais de um adesivo transdérmico para compensar o que esqueceu!
Se interromper o tratamento com Buprenorfina Teva
Se interromper ou finalizar o tratamento com este medicamento muito cedo, a dor pode voltar a aparecer. Se você deseja suspender o tratamento devido a efeitos adversos desagradáveis, consulte seu médico. Seu médico dirá o que você pode fazer e se pode ser tratado com outros medicamentos.
Algumas pessoas podem experimentar efeitos de abstinência após usar analgésicos potentes por muito tempo e deixar de usá-los. O risco de ter esses efeitos após suspender a aplicação dos adesivos de buprenorfina é muito baixo. No entanto, se você se sentir agitado, ansioso, nervoso ou tremendo, se estiver hiperativo, tiver dificuldade para dormir ou problemas digestivos, consulte seu médico.
Se tiver alguma outra dúvida sobre o uso deste medicamento, pergunte ao seu médico ou farmacêutico.
4. Possíveis efeitos adversos
Assim como todos os medicamentos, este medicamento pode produzir efeitos adversos, embora nem todas as pessoas os sofram.
Se você experimentar inflamação das mãos, pés, joelhos, face, lábios, boca ou garganta, que pode causar dificuldade para engolir ou respirar, habones urticariais, desvanecimento, cor amarelada da pele e olhos (também chamada de icterícia), remova o adesivo transdérmico e consulte seu médico ou vá ao hospital mais próximo imediatamente. Esses podem ser sintomas de uma reação alérgica grave muito rara.
Foram relatados os seguintes efeitos adversos:
Muito frequentes(podem afetar mais de 1 em cada 10 pessoas):
- náuseas (se sentir doente)
- eritema, prurido
Frequentes(podem afetar até 1 em cada 10 pessoas)
- tontura, dor de cabeça
- falta de ar
- vômitos, constipação
- mudanças na pele (exantema, geralmente por uso repetido), aumento da sudorese
- edema (tumefação das pernas), cansaço
Pouco frequentes(podem afetar até 1 em cada 100 pessoas)
- confusão, distúrbios do sono, inquietude
- diferentes graus de sedação (serenidade), que vão desde cansaço até confusão
- distúrbios circulatórios (tais como hipotensão ou raramente, perda de consciência devido à queda da pressão arterial)
- secura da boca
- erupções
- retenção urinária (menos urina do que o normal), alterações da micção
- fraqueza (abatimento)
Raros(podem afetar até 1 em cada 1.000 pessoas)
- perda de apetite
- ilusões como alucinações, ansiedade, pesadelos, diminuição do desejo sexual
- dificuldade de concentração, distúrbios da fala, confusão, alterações do equilíbrio, sensações anormais na pele (sensação de calor, formigamento ou entorpecimento)
- alterações da visão, visão borrada, inchaço das pálpebras
- sofocos
- dificuldade para respirar (depressão respiratória)
- azia estomacal
- habão urticarial
- dificuldades na ereção
- sintomas de abstinência, reações no local de administração
Muito raros(podem afetar até 1 em cada 10.000 pessoas)
- reações alérgicas graves
- dependência, mudanças de humor
- contração muscular, alterações do paladar
- pupilas pequenas
- dor de ouvido
- hiperventilação, soluço
- arcadas
- pústulas, vesículas
- dor no peito
Frequência não conhecida(a frequência não pode ser estimada a partir dos dados disponíveis)
- dermatite de contato (erupção cutânea com inflamação que pode incluir sensação de queimadura), descoloração da pele
Se você notar algum dos efeitos adversos mencionados anteriormente, consulte seu médico o mais rápido possível.
Em alguns casos, ocorrem reações alérgicas locais tardias com sinais marcados de inflamação. Nesses casos, o tratamento com Buprenorfina Teva deve ser interrompido após consulta ao médico.
Algumas pessoas podem ter sintomas de abstinência após usar analgésicos potentes por um período prolongado e deixar de usá-los. Após o tratamento com Buprenorfina Teva, o risco de sofrer sintomas de abstinência é baixo. No entanto, se você se sentir agitado, ansioso, nervoso ou tremendo, se estiver hiperativo, tiver dificuldade para dormir ou problemas digestivos, consulte seu médico.
Comunicação de efeitos adversos
Se você experimentar qualquer tipo de efeito adverso, consulte seu médico ou farmacêutico, mesmo que se trate de possíveis efeitos adversos que não aparecem neste prospecto. Você também pode relatá-los diretamente através do Sistema Espanhol de Farmacovigilância de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante a comunicação de efeitos adversos, você pode contribuir para fornecer mais informações sobre a segurança deste medicamento.
5. Conservação de Buprenorfina Teva
Mantenha este medicamento fora da vista e do alcance das crianças.
Não use este medicamento após a data de validade que aparece no frasco e no envelope após CAD. A data de validade é o último dia do mês que é indicado.
Este medicamento não requer condições especiais de conservação.
Conserve este medicamento em um local seguro e protegido, onde outras pessoas não possam acessá-lo. Ele pode causar danos graves e ser mortal para as pessoas que pudessem usá-lo de maneira acidental ou intencional quando não foi prescrito para elas.
Os medicamentos não devem ser jogados nos esgotos ou na lixeira. Deposite os frascos e os medicamentos que não são necessários no Ponto SIGRE da farmácia. Em caso de dúvida, pergunte ao seu farmacêutico como se livrar dos frascos e dos medicamentos que não são necessários. Dessa forma, você ajudará a proteger o meio ambiente.
6. Conteúdo do frasco e informações adicionais
Composição de Buprenorfina Teva
O princípio ativo é buprenorfina.
Cada adesivo transdérmico de 25 cm² contém 20 mg de buprenorfina e libera 35 microgramas de buprenorfina por hora.
Os demais componentes são:
- Matriz adesiva (com buprenorfina) povidona K90, ácido levulínico, oleil oleato, poli[ácido acrílico-co-butilacrilato-co-(2-etilhexil)acrilato-co-vinilacetato] (5:15:75:5)
- Matriz adesiva (sem buprenorfina): poli[(2-etilhexil)acrilato-co-glicidilmetacrilato-co-(2-hidroxietil)acrilato-co-vinilacetato] (68:0,15:5:27)
- Lâmina separadora entre as matrizes adesivas com e sem buprenorfina: lâmina de poli(etileno tereftalato).
- Camada de revestimento: poliéster.
- Lâmina protetora de liberação (na parte anterior cobrindo a matriz adesiva que contém buprenorfina): lâmina de poli(etileno tereftalato) siliconada.
- Tinta de impressão azul
Aspecto de Buprenorfina Teva e conteúdo do frasco
Adesivo retangular com bordas arredondadas de cor bege e impresso “Buprenorfina” e “35 μg / h”
Cada adesivo transdérmico está selado em um envelope à prova de crianças. Os adesivos estão disponíveis contendo 3, 4, 5, 6, 8, 10, 12, 16, 18 ou 20 adesivos transdérmicos.
Pode ser que apenas alguns tamanhos de frascos sejam comercializados.
Titular da autorização de comercialização
Teva Pharma S.L.U.
C/ Anabel Segura, 11 Edifício Albatros B, 1ª planta
28108 Alcobendas (Madrid)
Espanha
Responsável pela fabricação
Labtec GmbH
Heykenaukamp 10, Hamburgo
21147 Alemanha
ou
Merckle GmbH
Ludwig-Merckle-Straße 3, Blaubeuren
89143 Alemanha
ou
Teva Operations Poland Sp.z.o.o
ul. Mogilska 80, Krakow
31-546 Polônia
Este medicamento está autorizado nos Estados-Membros com os seguintes nomes:
Alemanha: | Buprenoratiopharm 35 Mikrogramm/Stunde Transdermales Pflaster |
Áustria: | Buprenorphin ratiopharm 35 Mikrogramm/h transdermales Pflaster |
Bélgica: | Buprenorphine Teva 35 microgram/u pleister voor transdermaal gebruik Buprenorphine Teva 35 microgrammes/h dispositif transdermique Buprenorphine Teva 35 Mikrogramm/S transdermales Pflaster |
Espanha: | Buprenorfina Teva 35 microgramos/hora adesivo transdérmico EFG |
Finlândia: | Buprenorphine ratiopharm 35 mikrog/tunti depotlaastari |
Croácia: | Laribon 35 mikrograma/h transdermalni flaster |
Países Baixos: | Buprenorfine Teva 35 microgram/uur pleister voor transdermaal gebruik |
Portugal: | Buprenorfina ratiopharm |
Reino Unido: | Timpron 35 micrograms/h Transdermal patch |
Data da última revisão deste prospecto:outubro 2024
A informação detalhada e atualizada deste medicamento está disponível na página da Agência Espanhola de Medicamentos e Produtos Sanitários (AEMPS) http://www.aemps.gob.es/
Você pode acessar informações detalhadas e atualizadas sobre este medicamento digitalizando com seu telefone móvel (smartphone) o código QR incluído no cartucho. Você também pode acessar essas informações no seguinte endereço de internet: https://cima.aemps.es/cima/dochtml/p/80609/P_80609.html
QR+URL

Quanto custa o BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico em Espanha em 2025?
O preço médio do BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico em dezembro de 2025 é de cerca de 21.53 EUR. Os valores podem variar consoante a região, a farmácia e a necessidade de receita. Confirme sempre com uma farmácia local ou fonte online para obter informações atualizadas.
- País de registo
- Preço médio em farmácia21.53 EUR
- Substância ativa
- Requer receita médicaSim
- Fabricante
- Esta informação é apenas para referência e não constitui aconselhamento médico. Consulte sempre um médico antes de tomar qualquer medicamento. A Oladoctor não se responsabiliza por decisões médicas baseadas neste conteúdo.
- Alternativas a BUPRENORFINA TEVA 35 microgramas/hora penso transdérmicoForma farmacêutica: PENSAMENTO TRANSDÉRMICO, 35 microgramas/horaSubstância ativa: buprenorphineFabricante: Andromaco Pharma S.L.Requer receita médicaForma farmacêutica: ADESIVO TRANSDÉRMICO, 52,5 microgramas/horaSubstância ativa: buprenorphineFabricante: Andromaco Pharma S.L.Requer receita médicaForma farmacêutica: PENSO TRANSDÉRMICO, 70 microgramas/horaSubstância ativa: buprenorphineFabricante: Andromaco Pharma S.L.Requer receita médica
Alternativas a BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico noutros países
As melhores alternativas com o mesmo princípio ativo e efeito terapêutico.
Alternativa a BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico em Poland
Alternativa a BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico em Ukraine
Médicos online para BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico
Avaliação de posologia, efeitos secundários, interações, contraindicações e renovação da receita de BUPRENORFINA TEVA 35 microgramas/hora penso transdérmico – sujeita a avaliação médica e regras locais.