
EMERADE 150 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG

Cómo usar EMERADE 150 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Emerade 150 microgramos solución inyectable en pluma precargada EFG
Emerade 300 microgramos solución inyectable en pluma precargada EFG
Emerade 500 microgramos solución inyectable en pluma precargada
adrenalina
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Emerade y para qué se utiliza
- Qué necesita saber antes de empezar a usar Emerade
- Cómo usar Emerade
- Posibles efectos adversos
- Conservación de Emerade
- Contenido del envase e información adicional
1. Qué es Emerade y para qué se utiliza
Emerade es un dispositivo autoinyector que contiene adrenalina en una solución para inyección en el músculo (intramuscular).
La adrenalina contrarresta la caída de la presión arterial en las reacciones anafilácticas. También estimula al corazón y facilita la respiración.
Emerade se utiliza como tratamiento de emergencia en las reacciones alérgicas graves (anafilaxia) causadas por alérgenos de las comidas, medicamentos, mordeduras o picaduras de insectos y otros alérgenos, así como en las desencadenadas por el ejercicio o por causas desconocidas.
2. Qué necesita saber antes de empezar a usar Emerade
Su médico le habrá explicado cuándo y cómo debe utilizar Emerade. Si no está usted completamente seguro o si tiene dudas, debe ponerse en contacto con su médico.
Advertencias y precauciones
Emerade siempre puede utilizarse durante una emergencia de tipo alérgico. Si usted es alérgico (hipersensible) al metabisulfito de sodio o a cualquiera de los demás componentes de Emerade, su médico le explicará en qué circunstancias debe utilizar Emerade.
Consulte a su médico antes de empezar a usar Emerade si usted presenta:
- una enfermedad cardiaca,
- presión arterial elevada,
- hiperfunción de la glándula tiroides,
- diabetes,
- un tumor de la glándula suprarrenal,
- aumento de la presión del ojo (glaucoma),
- reducción de la función renal,
- enfermedad de la próstata,
- niveles bajos de potasio o niveles altos de calcio en la sangre.
Si usted tiene asma podría presentar mayor riesgo de reacción alérgica grave.
Cualquier persona que haya sufrido un episodio de anafilaxia debe acudir al médico y hacerse pruebas de sustancias a las que podría ser alérgica, para poder evitarlas en el futuro. Es importante tener en cuenta que una alergia a una sustancia puede dar lugar a alergias a otras sustancias relacionadas.
Si usted presenta alergia a algún alimento es importante que compruebe la composición de todo lo que ingiera (incluidas las medicinas), ya que incluso cantidades pequeñas pueden causar reacciones graves.
También existe mayor riesgo de aparición de efectos adversos en personas en edad avanzada o en embarazadas.
Se deben seguir cuidadosamente las instrucciones de uso, para evitar una inyección accidental.
Emerade debe inyectarse por vía intramuscular en la parte externa del muslo. No se debe inyectar en la nalga debido al riesgo de inyección accidental en una vena.
Advertencias
La inyección accidental en las manos o pies puede dar lugar a reducción del riego sanguíneo en la zona afectada. Si se produce una inyección accidental en estas zonas, debe acudir inmediatamente al servicio de urgencias más cercano para recibir tratamiento.
Si tiene una capa de grasa subcutánea gruesa, existe el riesgo de que una sola dosis de Emerade no sea suficiente. Esto podría aumentar la necesidad de una segunda inyección de Emerade. Siga cuidadosamente las instrucciones de uso dadas en la sección 3.
Niños
Emerade no debe utilizarse en niños de menos de 15 kg.
En el caso de los niños menores de 15 Kg, no se puede administrar una dosis por debajo de 150 microgramos con suficiente precisión, y por tanto no se recomienda su uso a no ser que se trate de una situación potencialmente mortal y bajo asesoramiento médico.
Uso en deportistas:
Este medicamento contiene adrenalina, que puede producir un resultado positivo en las pruebas de control de dopaje.
Uso de Emerade con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Esto es particularmente importante si utiliza cualquiera de los siguientes fármacos:
- Antidepresivos como los antidepresivos tricíclicos o los inhibidores de la monoaminooxidasa (inhibidores de la MAO), ya que los efectos de la adrenalina pueden aumentar.
- Medicamentos para tratar la enfermedad de Parkinson, como los inhibidores de la catecol-O-metil transferasa (inhibidores de la COMT), ya que pueden aumentar el efecto de la adrenalina.
- Medicamentos que pueden hacer que el corazón tienda a presentar latidos irregulares (arritmias), como la Digitalis y la quinidina.
- Medicamentos denominados alfa o beta bloqueantes para enfermedades cardiacas o alteraciones del sistema nervioso, ya que pueden reducir el efecto de la adrenalina.
Los pacientes diabéticos deben controlar cuidadosamente sus niveles de glucosa tras el uso de Emerade, ya que la adrenalina puede aumentar el nivel de glucosa en sangre.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
La experiencia sobre la utilización de adrenalina durante el embarazo es limitada. No obstante, aunque estuviese embarazada no debe evitar el uso de Emerade en una situación de urgencia en la que su vida pueda estar en peligro.
Usted podrá amamantar a su hijo después de haber utilizado Emerade.
Conducción y uso de máquinas
Es poco probable que la capacidad para conducir y utilizar máquinas se vea afectada por la administración de una inyección de adrenalina, pero podría estar afectada por una reacción alérgica grave. Si dicha capacidad estuviese afectada, no debe conducir.
Emerade contiene metabisulfito de sodio
En raras ocasiones, el metabisulfito de sodio puede causar reacciones de hipersensibilidad graves o dificultad respiratoria (broncoespasmo). Si usted es alérgico (hipersensible) a metabisulfito de sodio, su médico le explicará en qué circunstancias debe utilizar Emerade.
Emerade contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Emerade
Asegúrese siempre de haber recibido formación en el uso de Emerade y siga exactamente las instrucciones de Emerade indicadas por su médico. En caso de duda, consulte a su médico o farmacéutico.
Compruebe la fecha de caducidad de sus autoinyectores de adrenalina y solicite a su médico o enfermera que le prescriba unos nuevos antes de que caduquen. Los inyectores caducados pueden no funcionar.
Emerade debe utilizarse inmediatamente si comenzasen los signos o síntomas de una reacción alérgica aguda (anafilaxia). Las reacciones pueden aparecer a los pocos minutos tras el contacto con el alérgeno y los síntomas pueden ser, por ejemplo, erupción en la piel, sofocos o hinchazón. Las reacciones más graves también afectan a la circulación sanguínea y la respiración.
Antes de que surja la necesidad de utilizar Emerade, asegúrese de que comprende en qué situaciones debe utilizarlo. Si presenta riesgo de anafilaxia, es importante que siempre lleve dos plumas de adrenalina con usted todo el tiempo. Emerade debe guardarse en la caja original, aunque durante el transporte por el paciente/cuidador es aceptable guardarlo en el envase especialmente diseñado en el que se entrega. Debe llevar siempre la pluma en este envase para asegurar la protección de la pluma y de la etiqueta que muestra cómo utilizarla en una situación de emergencia. Guarde siempre este folleto informativo en el estuche.
Posología
La dosis la decidirá su médico, que ajustará la dosis individualmente, por ejemplo dependiendo de su peso corporal.
Adultos
Adultos con menos de 60 Kg de peso
La dosis habitual es de 300 microgramos
Adultos con más de 60 Kg de peso
La dosis habitual es de 300 a 500 microgramos
Niños y adolescentes
No se recomienda el uso de Emerade 500 microgramos en niños.
Niños entre 15 y 30 kg de peso
La dosis habitual es de 150 microgramos.
Niños de más de 30 Kg de peso
La dosis habitual es de 300 microgramos.
Adolescentes de más de 30Kg de peso
Se deben seguir las recomendaciones indicadas para adultos.
Cómo se administra Emerade
Se deben seguir cuidadosamente las instrucciones de uso, para evitar una inyección accidental.
Se recomienda que sus familiares, cuidadores o profesores también reciban instrucciones sobre cómo administrar correctamente Emerade
Emerade solo debe utilizarse inyectándolo en la parte externa del muslo ante los primeros signos de una reacción alérgica grave. La inyección se administra al presionar Emerade contra el muslo. Se puede administrar atravesando la ropa. No se debe inyectar en la nalga (el trasero).
Si una pluma de adrenalina Emerade no se activa, inmediatamente se debe hacer un intento adicional usando una mayor fuerza al presionar la pluma contra el lugar de inyección previsto.
Si no tiene éxito, proceda inmediatamente a utilizar su segunda pluma.
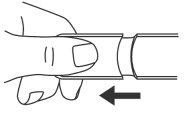
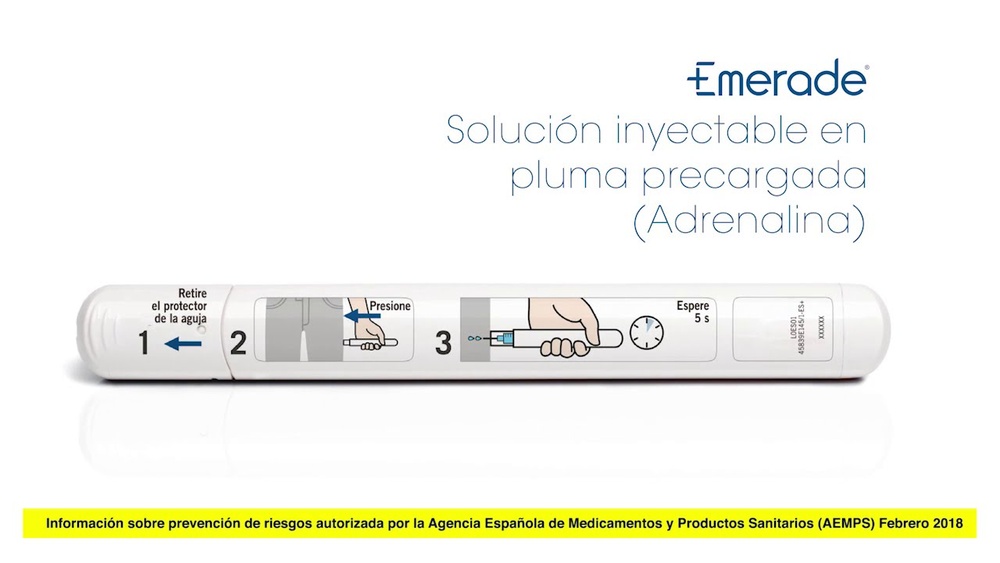
- Retire el tapón.
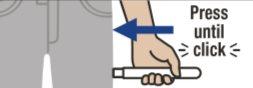 Sitúe Emerade contra la parte externa del muslo con un ángulo de 90º y presione firmemente para que el protector de la aguja se retraiga. Oirá un "clic" cuando el dispositivo se active y la aguja penetre en el muslo.
Sitúe Emerade contra la parte externa del muslo con un ángulo de 90º y presione firmemente para que el protector de la aguja se retraiga. Oirá un "clic" cuando el dispositivo se active y la aguja penetre en el muslo.
y
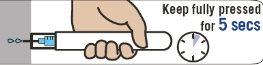 Sujete Emerade completamente presionado contra el muslo durante 5 segundos. A continuación masajee ligeramente la zona de la inyección.
Sujete Emerade completamente presionado contra el muslo durante 5 segundos. A continuación masajee ligeramente la zona de la inyección.
- Busque atención médica inmediatamente.
La aguja de Emerade está protegida antes, durante y después de la inyección.
Cuando se completa la inyección, el protector de la aguja de Emerade aparece visiblemente más largo y el émbolo está visible en la ventana de inspección al levantar la etiqueta.
Mensajes claves para los pacientes:
- A veces, una sola dosis de adrenalina no es suficiente para contrarrestar totalmente los efectos de una reacción alérgica grave. Por esta razón, su médico deberá prescribirle dos plumas Emerade.
- Si sus síntomas no han mejorado o han empeorado en los 5 - 15 minutos después de la primera inyección, bien usted o la persona con quien esté debe administrarle una segunda. Por esta razón, debe llevar dos plumas de Emerade con usted todo el tiempo.
- Emerade está diseñado como tratamiento de emergencia. Inmediatamente después de la utilización de Emerade siempre se debe buscar atención médica. Pida a alguien que permanezca con usted hasta que llegue la ambulancia, por si vuelve a sentirse mal.
- Llame al 112, pida una ambulancia e indique que es un caso de 'anafilaxia’,incluso aunque parezca que los síntomas están mejorando. Deberá acudir al hospital para su observación o para recibir más tratamiento, según sea necesario. Esto se debe a que cierto tiempo después puede volver a aparecer la reacción. Lleve con usted la pluma utilizada.
- Mientras espera a la ambulancia debe permanecer tumbado con los pies elevados, a no ser que esta posición no le permita respirar, en cuyo caso deberá sentarse erguido.
- Los pacientes inconscientes deben colocarse de lado, en posición de recuperación.
Tras la utilización de la pluma de Emerade siguiendo las instrucciones, el paciente puede verificar si la pluma se ha activado. Las imágenes de debajo (Fig.1-Fig.2) se refieren a todas las dosis de Emerade (150 microgramos, 300 microgramos y 500 microgramos).
La pluma Emerade sin usar (antes de la activación) tiene el protector de aguja en su posición normal (Fig. 1).
Fig 1.
La pluma de Emerade que se ha activado tendrá el protector de la aguja extendido (Fig. 2).

Fig. 2
Si el protector de aguja no está extendido, la pluma no se ha activado.
Una pluma de Emerade que se ha activado y, ha administrado con éxito una dosis de adrenalina, mostrará un émbolo coloreado en la ventana de inspección (que se revela al despegar la etiqueta de la pluma):
150 microgramos: amarillo
300 microgramos: verde
500 microgramos: azul.
Si la ventana de inspección sigue mostrando un líquido transparente (solución de adrenalina), la pluma no ha administrado correctamente una dosis de adrenalina. La flecha en la etiqueta de la pluma indica dónde se puede levantar la etiqueta para revelar la ventana de inspección.
No retire el tapón a no ser que sea necesario administrar la inyección.
Tras la inyección puede que quede algo de líquido en el autoinyector. El autoinyector no se puede reutilizar.
Hay autoinyectores sin aguja (plumas para demostración) disponibles para formación.
Por favor consulte con su médico.
Si usa más Emerade del que debe
Si se administra una dosis mayor o si se inyecta Emerade accidentalmente en un vaso sanguíneo o en la mano, deberá buscar atención médica inmediatamente.
Su presión sanguínea podría elevarse de forma brusca. La sobredosis puede causar un aumento repentino de la presión sanguínea, latido cardiaco irregular y acumulación de líquido en los pulmones, lo cual puede dificultar la respiración.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 915620420, indicando el medicamento y la cantidad ingerida.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Los siguientes efectos adversos se basan en la experiencia con el uso de adrenalina, aunque no se puede estimar su frecuencia:
- problemas cardiacos, como latido cardiaco rápido e irregular, dolor torácico,
- elevación de la presión sanguínea, estrechamiento de los vasos sanguíneos,
- sudoración,
- náuseas, vómitos,
- dificultad para respirar,
- dolor de cabeza, mareos,
- debilidad, temblor,
- ansiedad, alucinaciones,
- desmayo,
- cambios en los valores en sangre, como aumento del azúcar en sangre, reducción del potasio y mayor acidez
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano https://www.notificaRAM.es
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Emerade
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el envase protector de plástico en el que se entrega. El envase de plástico que contiene la pluma/plumas puede guardarse en la caja exterior.
Conservar por debajo de 25 ºC. No congelar.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja exterior. La fecha de caducidad es el último día del mes que se indica. Tras la fecha de caducidad, deseche Emerade y sustitúyalo por otro envase. Examine periódicamente la solución a través de la ventana de inspección levantando la etiqueta para asegurarse de que la solución sigue siendo transparente e incolora. No utilice este medicamento si observa que la solución ha cambiado de color o contiene algún precipitado.
Revise el autoinyector si se le cayese. Sustitúyalo si observa algún daño o fuga.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional.
Composición de Emerade
El principio activo es adrenalina en forma de tartrato.
Emerade 150 microgramos libera 150 microgramos de adrenalina en 0,15 ml de solución.
Emerade 300 microgramos libera 300 microgramos de adrenalina en 0,3 ml de solución.
Emerade 500 microgramos libera 500 microgramos de adrenalina en 0,5 ml de solución.
Los demás componentes son: cloruro de sodio, metabisulfito de sodio (E223), edetato de disodio, ácido clorhídrico y agua para preparaciones inyectables.
Aspecto de Emerade y contenido del envase
Emerade es un autoinyector que libera una única dosis de adrenalina. Emerade contiene una solución para inyección, transparente e incolora, dentro de una jeringuilla de cristal. Emerade no contiene látex.
El dispositivo es un cilindro blanco con un protector que cubre la aguja y con un mecanismo de gatillo.
Longitud de la aguja expuesta:
Emerade 150 microgramos: 16mm
Emerade 300 microgramos y 500 microgramos: 23mm.
Tamaños de envase: 1 o 2 plumas precargadas.
Puede que no todos los tamaños de envase estén comercializados.
Titular de la autorización de comercialización y responsable de la fabricación
Titular
PharmaSwiss Ceská republika s.r.o.
Jankovcoca 1569/2c, 170 00 Praga 7.
República Checa.
Responsable de la Fabricación
Rechon Life Science AB
Soldattorpsvägen 5, SE-216 13 Limhamm.
Suecia
Fecha de la última revisión de este prospecto: Diciembre 2021
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es/
Puede acceder a información detallada y actualizada sobre este medicamento escaneando con su teléfono móvil (smartphone) el código QR incluido en el prospecto. También puede acceder a esta información en la siguiente dirección de internet:
https://cima.aemps.es/info/80146
https://cima.aemps.es/info/80147
https://cima.aemps.es/info/80149

- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EMERADE 150 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA EFGForma farmacéutica: INYECTABLE, 1 mg/10 mlPrincipio activo: AdrenalinaFabricante: Laboratoire AguettantRequiere recetaForma farmacéutica: INYECTABLE, Adrenalina base 1 mg/mlPrincipio activo: AdrenalinaFabricante: B Braun Medical S.A.Requiere recetaForma farmacéutica: INYECTABLE, 1 mg/mlPrincipio activo: AdrenalinaFabricante: Laboratorios Basi Industria Farmaceutica S.A.Requiere receta
Médicos online para EMERADE 150 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EMERADE 150 MICROGRAMOS SOLUCION INYECTABLE EN PLUMA PRECARGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











