
PROCRIN SEMESTRAL 30 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE

How to use PROCRIN SEMESTRAL 30 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Procrin Semestral 30 mg powder and solvent for injectable suspension in a pre-filled syringe
Leuprorelin acetate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
|
Contents of the package leaflet:
- What is Procrin Semestral and what is it used for
- What you need to know before you use Procrin Semestral
- How to use Procrin Semestral
- Possible side effects
- Storage of Procrin Semestral
- Contents of the pack and further information
1. What is Procrin Semestral and what is it used for
Procrin Semestral is a medicine that belongs to a group of gonadotropin-releasing hormone analogues and is used to reduce circulating testosterone and estrogen levels in the body.
Procrin Semestral is indicated for the palliative treatment of advanced hormone-dependent prostate cancer and for the treatment of high-risk and locally advanced hormone-dependent prostate cancer in combination with radiotherapy.
Procrin Semestral should be administered to patients who have previously been treated with one of the following medications: natural gonadotropin-releasing hormone analogues (GnRH or LH-RH) and/or antiandrogens.
2. What you need to know before you use Procrin Semestral
Do not use Procrin Semestral:
- If you are allergic to leuprorelin acetate or similar nonapeptides, or to any of the other components of this medicine (listed in section 6).
- After surgical removal of your testicles.
- As the only treatment, if you have symptoms related to pressure on the spinal cord or a tumor in the spine.
- If you are a woman or child.
This is a medicine that is only indicated for men, but as the same active ingredient is used in other doses in women, it is important to note that it should not be used in women:
- If you have undiagnosed vaginal bleeding.
- If you are pregnant or think you may be pregnant during treatment.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with Procrin Semestral
- Generally, there is an increase in blood levels of the male sex hormone (testosterone) during the first week of treatment. This can lead to a temporary worsening of symptoms related to the disease and also to the appearance of new symptoms that had not been experienced until then. These symptoms include especially bone pain, urinary disorders, and pressure on the spinal cord. These symptoms usually subside as treatment continues. If the symptoms do not subside or worsen, you should contact your doctor immediately.
- If you experience sudden headache, vomiting, visual disturbances, or changes in mental status in the first few weeks of treatment, go to your doctor immediately, as you may be experiencing a serious condition called hypophysial apoplexy (a disease caused by decreased blood flow to an area of the brain).
- If you experience urinary tract obstruction, presence of blood in the urine, or vertebral and/or cerebral metastatic lesions. In these cases, your doctor should monitor you frequently and also evaluate the possibility of starting treatment with the daily preparation of PROCRIN during the first two weeks of treatment to facilitate discontinuation if necessary.
- It can cause loss of bone mineral density with a risk of fractures due to osteoporosis.
- Seizures can occur in patients predisposed to them due to their underlying disease, in patients receiving medications that can cause seizures, and to a lesser extent in patients not included in either of the above groups.
- If you are diabetic, as treatment with leuprorelin can affect glucose control.
- If you have or have had heart problems, inform your doctor.
- If you were born with or have a history of prolonged QT interval on the electrocardiogram (record of the heart's electrical activity), electrolyte imbalance, or take medications that can cause changes in the electrocardiogram (see Use of other medications). Inform your doctor.
- Tell your doctor if you have any heart or blood vessel condition or are being treated for it, including medications to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using Procrin Semestral.
- Depression has been reported in patients using Procrin, which can be severe. If you are using Procrin and have a depressed mood, inform your doctor.
- If you have a history of hyperglycemia, diabetes, hypercholesterolemia, and/or fatty liver, your doctor should closely monitor you to detect possible changes/metabolic syndrome.
- If you have fatty liver
Severe skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), have been reported in association with leuprorelin. Discontinue use of leuprorelin and seek medical attention immediately if you notice any symptoms related to these severe skin reactions described in section 4.
Use of Procrin Semestral with other medications
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medication.
Procrin Semestral may interfere with some medications used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medications, e.g., methadone (used for pain relief and detoxification from other medications), moxifloxacin (an antibiotic), antipsychotics used to treat severe mental illnesses).
Interference with laboratory tests:
Changes in the results of various laboratory tests may occur; it is normal for these fluctuations to occur as treatment progresses, and it is the doctor who should evaluate this behavior.
Pregnancy, breastfeeding, and fertility
This medicine is intended exclusively for male patients.
If Procrin Semestral is accidentally administered to women, it should be noted that Procrin Semestral is contraindicated in pregnant women or those who may be pregnant and during the lactation period. There is a possibility of spontaneous abortion if administered during pregnancy.
Consult your doctor or pharmacist before using any medication.
Driving and using machines
The ability to drive and use machines may be impaired due to fatigue, dizziness, vertigo, and vision disorders, which can be possible adverse reactions to treatment or a consequence of the underlying disease.
Use in athletes:
This medicine contains leuprorelin acetate, which can produce a positive result in doping tests.
3. How to use Procrin Semestral
Procrin Semestral should only be administered by your doctor or nurse. They will be responsible for preparing the product.
Procrin Semestral is administered subcutaneously (injection of the medicine into the tissue located immediately under the skin).
The normal dose is a single subcutaneous injection every six months.
Your doctor will decide what dose of Procrin Semestral you should receive and when you should receive it. You should receive the dose specified by your doctor.
Your doctor may perform blood tests to check the effect of Procrin Semestral.
In some people, the doctor may also prescribe a medication belonging to the group of antiandrogens to control the symptoms caused by the elevation of testosterone levels (male sex hormone) in the first few weeks of treatment.
If you think the action of Procrin Semestral is too strong or too weak, tell your doctor or pharmacist.
If you use more Procrin Semestral than you should
There is no clinical experience with the effects of an acute overdose of leuprorelin acetate. In animal studies, it was observed that administration of doses approximately 133 times higher than recommended for human use resulted in dyspnea (shortness of breath), decreased activity, and local irritation at the injection site. In case of overdose, the patient should be monitored and accompanied.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91.562.04.20, indicating the medication and the amount administered.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Most of the adverse effects observed with leuprorelin acetate are due to the medicine's own action, which produces increases and/or decreases in male hormone levels.
Adverse Effects at the Start of Treatment:
During clinical trials in prostate cancer, a transient increase in testosterone (male hormone) in the blood appears at the beginning of treatment in patients who have not been previously treated with hormonal therapy, which is occasionally associated with a worsening of signs and symptoms, generally a transient increase in bone pain. In patients with vertebral metastases and/or urinary obstruction or blood in the urine, neurological problems such as temporary weakness and/or abnormal sensation of tingling, cold, itching, etc. in the legs or worsening of urinary symptoms may appear. If the presence of these symptoms seems important to you, inform your doctor.
The adverse effects collected during clinical trials and after the marketing of the medicine are presented below, classified according to frequency:
Very Common (may affect more than 1 in 10 people):
Erythema (superficial skin inflammation, characterized by red spots), erythema at the injection site.
Common (may affect up to 1 in 100 people):
Exacerbation of prostate tumor, worsening of prostate tumor, anemia, increased appetite, diabetes mellitus (excess glucose in the blood), glucose tolerance disorder, increased fat in the blood (increased cholesterol), increased low-density lipoproteins (LDL) in the blood, increased triglycerides (a type of fat), decreased sexual desire, increased sexual desire, mood changes, depression, headache, dilation of arteries or veins, hot flashes, hypotension (low blood pressure), sudden decrease in blood pressure when standing up (orthostatic hypotension), abnormal accumulation of fluid in the lungs, excessive sweating (hyperhidrosis), dry skin, skin rash, urticaria, abnormal hair growth, hair disorder, night sweats, hair loss, skin pigmentation disorder, cold sweat, excessive hair growth on the face or body (hirsutism), muscle weakness, osteoporosis (disease in which bone mineral density is low and bones may have fractures or microfractures), erectile dysfunction, testicular atrophy, breast growth in males, breast congestion, testicular pain, breast growth and enlargement, breast pain, prostate pain, penile inflammation, penile disorder, feeling of fatigue, reaction at the injection site, inflammation at the injection site, pain at the injection site, induration at the injection site, abscess at the injection site, dry mucous membranes, increased transaminases, weight gain or loss, increased prostate-specific antigen (PSA), decreased bone density.
1Adverse effects frequently associated with the medicine's own pharmacological action.
2Adverse effects frequently associated with the medicine's own pharmacological action in prolonged treatments (6 to 12 months).
Uncommon (may affect up to 1 in 1000 people):
Abnormal weight gain, sleep disorders, mood changes, depression, dizziness, heart failure (the heart loses its ability to pump blood effectively), nausea, itching, night sweats, partial hair loss, increased urination, difficulty urinating, peripheral edema (fluid accumulation in ankles, feet, and legs), abnormal liver function test.
3Adverse effects frequently associated with the medicine's own pharmacological action in short treatments.
Frequency Not Known (cannot be estimated from the available data):
Infection, urinary tract infection, pharyngitis, pneumonia, skin cancer, anaphylactic reaction (generalized immune reaction, usually severe), goiter (enlargement of the thyroid gland), pituitary apoplexy (death of an area of pituitary gland tissue), low blood sugar levels, dehydration, high phosphorus levels in the blood, decreased protein levels in the blood, nervousness, insomnia, anxiety, delirious ideas, suicidal thoughts, attempted suicide, dizziness, numbness or tingling in some parts of the body with a sensation of tingling (paresthesia), lethargy, memory impairment, taste disturbance (dysgeusia), hypoesthesia (decreased sensitivity), fainting, peripheral neuropathy (disorder of the peripheral nerves), stroke, loss of consciousness, transient ischemic attack, paralysis, neuromuscular disorders, convulsions, blurred vision, eye disorders, decreased vision, decreased vision in one or both eyes (amblyopia), dry eyes, tinnitus, decreased hearing, congestive heart failure (the heart loses its ability to pump blood effectively), altered heart rhythm, myocardial infarction, angina pectoris, increased heart rate, slow heart rate, sudden cardiac death, changes in the electrocardiogram (ECG) (prolongation of the QT interval), lymphatic fluid accumulation in tissues (lymphedema), hypertension (high blood pressure), vein inflammation caused by a blood clot (phlebitis), blockage of an artery by a blood clot (thrombosis), varicose veins (swollen and painful veins due to abnormal blood accumulation), pleural rub (noise produced by the contact of the two inflamed pleural leaves), lung tissue scarring or thickening (pulmonary fibrosis), nosebleeds, breathing difficulties, coughing up blood, cough, pleural effusion (fluid in the chest), lung infiltrate, respiratory disorders, sinus congestion, pulmonary embolism, inflammatory disorder of the lower airways (interstitial lung disease), constipation, vomiting, gastrointestinal bleeding, inflamed abdomen, diarrhea, difficulty swallowing, dry mouth, duodenal ulcer, gastrointestinal disorder, peptic ulcer, rectal polyps, abnormal liver function, yellow skin and eyes (jaundice), non-alcoholic fatty liver, severe liver damage, hair loss, red spots on the skin (ecchymosis), photosensitivity reaction, urticaria, dermatitis, skin lesions, erythema multiforme, bullous dermatitis, exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, muscle pain, bone inflammation, joint disorders, joint pain, ankylosing spondylitis (inflammatory disease affecting the vertebrae), tenosynovitis (inflammation of the tendon sheath), urinary incontinence, sudden and powerful urge to urinate, presence of blood in the urine, bladder spasms, urinary tract disorders, urinary tract obstruction, breast growth in males, testicular disorder, general pain, edema (swelling), general fatigue, essential fever (pyrexia), sterile abscess at the injection site, hematoma at the injection site, chills, nodule, thirst, inflammation, pelvic fibrosis, increased urea in the blood, increased uric acid in the blood, increased creatinine in the blood, increased calcium in the blood, abnormal electrocardiogram (ECG), signs of myocardial ischemia on the electrocardiogram, decreased platelets, decreased potassium in the blood, increased white blood cells, decreased white blood cells, increased prothrombin time, increased activated partial thromboplastin time, heart murmur (noise indicating heart malfunction), increased low-density lipoproteins in the blood, increased triglycerides in the blood, increased bilirubin in the blood, spinal fracture.
Seek immediate medical attention if you notice any of the following symptoms:
If you notice that you have circular or target-shaped red spots on your trunk, often with central blisters, skin peeling, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin rashes may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome/toxic epidermal necrolysis).
Redness of the skin and rash with itching (toxic skin rash)
A skin reaction that causes grains or red spots on the skin, which may look like a target, with a dark red center surrounded by lighter red rings (erythema multiforme).
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Procrin Semestral
Keep out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging after CAD. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Medicines should not be thrown away through the sewage system or in the trash. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Procrin Semestral
The active ingredient is leuprorelin acetate. Each preloaded syringe contains 30 mg of leuprorelin (as acetate) equivalent to 28.58 mg of leuprorelin.
The other components are:
Powder: Polylactic acid and mannitol.
Solvent: Sodium carboxymethylcellulose, mannitol, polysorbate 80, and water for injectable preparations.
1B
Each package contains a preloaded syringe with a double chamber with a 23G needle for single administration, a plunger, and an alcohol-impregnated swab.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
AbbVie Spain S.L.U.,
Avenida de Burgos 91,
28050 Madrid,
Spain.
Manufacturer
ABBVIE LOGISTICS B.V.
Zuiderzeelaan 53 8017 Zwolle,
Netherlands
or
AbbVie Deutschland GmbH&Co.KG,
Knollstrasse,
67061 Ludwigshafen,
Germany
Date of the Last Revision of this Prospectus: February 2025
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
2B
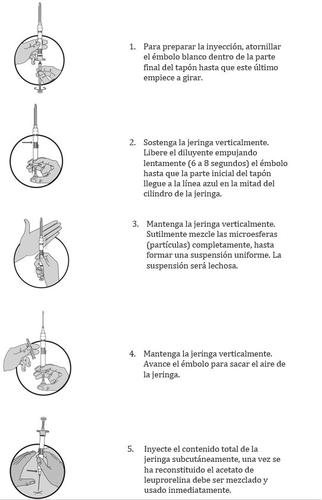
Instructions for the Correct Administration of the Preparation:
- Country of registration
- Average pharmacy price383.71 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROCRIN SEMESTRAL 30 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION IN PRE-FILLED SYRINGEDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 45 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: leuprorelinManufacturer: Recordati Industria Chimica E Farmaceutica S.P.A.Prescription required
Online doctors for PROCRIN SEMESTRAL 30 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE
Discuss questions about PROCRIN SEMESTRAL 30 mg POWDER AND SOLVENT FOR INJECTABLE SUSPENSION IN PRE-FILLED SYRINGE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












