
Eligard 7,5 mg

Ask a doctor about a prescription for Eligard 7,5 mg

How to use Eligard 7,5 mg
Package Leaflet: Information for the User
Eligard 7.5 mg,
powder and solvent for solution for injection
Leuprolide acetate
Read all of this leaflet carefully before using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. See section 4.
Contents of the pack
- 1. What Eligard is and what it is used for
- 2. Before you use Eligard
- 3. How to use Eligard
- 4. Possible side effects
- 5. How to store Eligard
- 6. Contents of the pack and other information
1. What Eligard is and what it is used for
Eligard contains the active substance leuprolide acetate, which belongs to a group of medicines called gonadorelin analogues. These medicines are used to stop the production of certain sex hormones (testosterone).
Eligard is used in adult men for the treatment of prostate cancerwith metastases, which is sensitive to hormone therapy, and in combination with radiotherapy - for the treatment of high-risk prostate cancer without metastases, which is sensitive to hormone therapy.
2. Before you use Eligard
When not to use Eligard
- In women and children.
- If you are allergic to the active substance leuprolide acetate, or to any other medicine of this type (gonadorelin) or to any of the other ingredients of Eligard (listed in section 6).
- After surgical removal of the testes, as Eligard will not cause further reduction in testosterone levels in the blood.
- As the only treatment if you have symptoms related to compression of the spinal cord or metastases to the spine. In such cases, Eligard can only be used in combination with other medicines used to treat prostate cancer.
Warnings and precautions
Before starting treatment with Eligard, discuss with your doctor, pharmacist, or nurse:
- If you have any heart or blood vessel disease, including irregular heart rhythm (arrhythmia) or if you are taking medicines for these conditions. The risk of irregular heart rhythm may increase during treatment with Eligard.
or if you have difficulty urinating. In this case, your condition should be monitored during the first weeks of treatment.
- If compression of the spinal cord or difficulty urinating worsens. During concurrent use of medicines with similar action to Eligard, severe cases of spinal cord compression and ureteral obstruction have been observed, which can cause symptoms such as paralysis. In such cases, standard treatment should be applied.
- If you experience sudden headache, vomiting, change in mental status, or circulatory collapse within two weeks of Eligard administration. In such cases, you should immediately inform your doctor or medical staff. These are symptoms of a rare condition called pituitary apoplexy, which has been reported in association with the use of other medicines with similar action to Eligard.
- If you have diabetes(high blood sugar levels). In this case, your condition should be monitored during treatment.
- Treatment with Eligard may increase the risk of fractures due to osteoporosis (decreased bone density).
- There have been reports of depression in patients taking Eligard. If you experience depressive moods while taking Eligard, you should inform your doctor.
- There have been reports of cardiovascular diseases in patients taking medicines similar to Eligard - it is not known whether their occurrence is related to the use of these medicines. If you experience symptoms of cardiovascular disease while taking Eligard, you should inform your doctor.
- There have been reports of seizures in patients who have been administered Eligard. If you experience seizures while taking Eligard, you should inform your doctor.
- If you experience severe or recurring headaches, vision problems, or ringing in the ears, you should immediately consult your doctor.
- If you have a fatty liver
In association with the use of leuprolide, severe skin reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), have been reported. If you notice any symptoms related to severe skin reactions described in section 4, you should discontinue leuprolide and immediately consult your doctor.
Complications occurring in the initial period of Eligard use
During the first week of treatment, a temporary increase in testosterone levels in the blood is observed, which may lead to transient worseningof disease symptoms or the appearance of new ones that have not occurred before. These symptoms include, in particular, bone pain, urinary disorders, spinal cord compression, and hematuria (blood in the urine). These symptoms usually resolve during continued treatment. If the symptoms do not resolve, you should consult your doctor.
Lack of Eligard effect
In some patients, tumors that are not sensitive to the reduction of testosterone levels in the blood are found. If you feel that Eligard is not having sufficient effect, you should inform your doctor.
Eligard and other medicines
Eligard may affect the action of certain medicines used to treat heart rhythm disorders (e.g., quinidine, procainamide, amiodarone, and sotalol) or increase the risk of heart rhythm disorders when used with certain other medicines (e.g., methadone (used for pain relief and detoxification in drug addicts), moxifloxacin (an antibiotic), and antipsychotic medicines used to treat severe mental illnesses).
You should inform your doctor or pharmacist about all medicines you are currently taking or have recently taken, including those that are available without a prescription.
Pregnancy and breastfeeding
Eligard is contraindicated in women.
Driving and using machines
Fatigue, dizziness, and vision disturbances may be side effects of Eligard or may result from the underlying disease. If you experience any of these side effects, you should exercise caution when driving or operating machinery.
3. How to use Eligard
Dosage
This medicine should always be used as directed by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist.
If your doctor does not instruct otherwise, Eligard 7.5 mg is administered once a month.
The injected solution forms a reservoir of the active substance, from which the active substance - leuprolide acetate - is continuously released over one month.
Additional tests
The response to Eligard treatment should be monitored by your doctor based on clinical symptoms and prostate-specific antigen (PSA) levels in the blood.
Method of administration
Eligard should only be administered by a doctoror nurse, who will also prepare the product.
After preparation, Eligard is administered as a subcutaneous injection (injection into the tissue under the skin). You should absolutely avoid intravenous (into a vein) or intra-arterial (into an artery) injection. As with other active substances used in subcutaneous injections, the injection sites should be periodically changed.
Use of a higher than recommended dose of Eligard
The medicine is usually administered by a doctor or appropriately trained medical staff, so it is unlikely that a higher dose of the medicine will be administered.
If a higher dose of the medicine is administered, your doctor will monitor your condition and provide appropriate treatment if necessary.
Missed dose of Eligard
If it is suspected that a monthly dose of the medicine has been missed, you should inform your doctor.
Discontinuation of Eligard treatment
Treatment of advanced prostate cancer requires long-term administration of Eligard.
Therefore, you should not discontinue treatment even if your condition improves or the symptoms of the disease disappear.
If treatment is discontinued prematurely, the symptoms of the disease may worsen.
Do not discontinue treatment without prior consultation with your doctor.
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Eligard can cause side effects, although not everybody gets them.
The side effects observed during treatment with Eligard are mainly due to the specific action of leuprolide acetate, which increases or decreases the levels of certain hormones. The most commonly observed side effects are hot flashes (in about 58% of patients), nausea, malaise, and fatigue, as well as transient reactions at the injection site.
Side effects in the initial period of treatment
During the first weeks of treatment with Eligard, there may be a worsening of disease symptoms due to the initial, temporary increase in testosterone levels in the blood. Therefore, your doctor may recommend taking an anti-androgen (a substance that reduces the action of testosterone) during the initial phase of treatment to reduce this effect of the medicine (see also section 2. Important information before using Eligard; Complications occurring in the initial period of Eligard use).
Local side effects
Local side effects described after injection of Eligard are those that frequently occur after subcutaneous injection (injection into the tissue under the skin) of similar medicines. Mild burning sensation immediately after injection is very common. Stinging and pain after injection, as well as bruising at the injection site, are common. Redness at the injection site has been reported frequently. Hardening of tissue and ulceration at the injection site are uncommon. The above-mentioned local side effects that occur after subcutaneous injection are mild and described as short-term. They do not recur between consecutive injections.
Very common side effects (may affect more than 1 in 10 people)
- Hot flashes
- Spontaneous bleeding from the skin and mucous membranes, redness of the skin
- Fatigue, reactions at the injection site (see above:Local side effects)
Common side effects (may affect up to 1 in 10 people)
- Nasopharyngitis (common cold symptoms)
- Nausea, malaise, diarrhea, gastritis (stomach and intestine/colon inflammation)
- Pruritus, night sweats
- Arthralgia
- Urinary frequency (need to urinate more often), difficulty urinating, painful urination, decreased urine output
- Breast tenderness, breast enlargement, testicular atrophy, testicular pain, infertility, erectile dysfunction, decreased penis size
- Chills (episodes of increased shivering with high fever), weakness
- Prolonged bleeding time, changes in blood parameter values, decreased red blood cell count/anemia
Uncommon side effects (may affect up to 1 in 100 people)
- Urinary tract infections, local skin infections
- Worsening of diabetes symptoms
- Unusual dreams, depression, decreased libido
- Dizziness, headache, sensory disturbances, insomnia, taste disturbances, olfactory disturbances
- Hypertension (high blood pressure), hypotension (low blood pressure)
- Shortness of breath
- Constipation, dry mouth, dyspepsia (indigestion with a feeling of fullness in the stomach, stomach pain, belching, nausea, vomiting, heartburn), vomiting
- Increased skin moisture, excessive sweating
- Back pain, muscle spasms
- Hematuria (blood in the urine)
- Urinary retention, frequent urination, inability to urinate
- Breast enlargement in men, impotence
- Lethargy (drowsiness), pain, fever
- Weight gain
- Loss of balance, feeling of emptiness in the head
- Muscle atrophy/muscle loss after long-term use
Rare side effects (may affect up to 1 in 1,000 people)
- Involuntary movements
- Sudden loss of consciousness, fainting
- Bloating with gas, belching
- Hair loss, skin rashes (pustules on the skin)
- Chest pain
- Ulceration at the injection site
Very rare side effects (may affect up to 1 in 10,000 people)
- Necrosis at the injection site
Frequency not known (cannot be estimated from the available data)
- Changes in electrocardiogram (QT interval prolongation)
- Pneumonia, lung disease
- Idiopathic intracranial hypertension (increased pressure inside the skull, characterized by headache, double vision, and other vision-related symptoms, as well as ringing in one or both ears)
- Red, flat, round, or oval patches on the torso, often with blisters in the center, peeling skin, ulcers in the mouth, throat, nose, genitals, and eyes. These severe skin reactions may be preceded by fever and flu-like symptoms (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- Redness of the skin and itchy rash (toxic skin eruptions)
- Skin reaction causing the appearance of red dots or patches on the skin, which may resemble a target with a dark red center surrounded by lighter red rings (erythema multiforme)
Other side effects
Other side effects described in the literature as associated with leuprolide treatment - the active substance contained in Eligard - include peripheral edema (fluid accumulation in tissues, manifested as swelling of the hands and feet), pulmonary embolism (manifested as shortness of breath, difficulty breathing, and chest pain), palpitations (perceptible heartbeat), decreased muscle strength, chills, rash, and memory and vision disturbances. With long-term treatment with Eligard, an increased frequency of osteoporosis (decreased bone density) symptoms can be expected, which increases the risk of fractures.
Rarely, severe allergic reactions have been reported after the use of products belonging to the same group as Eligard, causing difficulty breathing or dizziness.
Seizures have been reported after the use of products belonging to the same group as Eligard.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should inform your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Eligard
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Storage instructions
Store in a refrigerator (2°C to 8°C).
Store in the original package to protect from moisture.
Before administration, the medicinal product must be at room temperature. Remove it from the refrigerator approximately 30 minutes before preparation. After removal from the refrigerator, the product can be stored in the original package at room temperature (below 25°C) for up to 4 weeks.
After the first opening of the plastic tray sealed with foil, the solution should be prepared immediately and administered to the patient as soon as possible. The product is for single use only.
Instructions for disposal of unused or expired Eligard
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Eligard contains
The active substance of Eligard is leuprolide acetate.
One pre-filled syringe (Syringe B) contains 7.5 mg of leuprolide acetate.
The other ingredients of the medicine are poly(DL-lactic-co-glycolic acid) (50:50) and N-methyl-2-pyrrolidone contained in the pre-filled syringe with solvent for solution for injection (Syringe A).
What Eligard looks like and contents of the pack
Eligard is a powder and solvent for solution for injection.
Eligard 7.5 mg is available in the following packs:
- A set consisting of a thermoformed tray sealed with foil and a sterile needle with a 20G diameter, in a cardboard box. The tray contains a pouch with a desiccant and a system of connected syringes consisting of: Syringe A containing solvent and Syringe B containing powder, a connector with a latch for Syringes A and B.
- A multipack containing 3 sets, each containing one system of connected syringes.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Recordati Industria Chimica e Farmaceutica S.p.A.
Via Matteo Civitali 1
20148 Milan
Italy
For more information, contact your local representative of the marketing authorization holder:
Recordati Polska sp. z o.o.
Al. Armii Ludowej 26
00-609 Warsaw
phone: +48 22 206 84 50
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria:
Eligard Depot 7.5 mg
Belgium:
Depo-Eligard 7.5 mg
Cyprus:
Eligard
Czech Republic:
Eligard
Denmark:
Eligard
Estonia:
Eligard
Finland:
Eligard
France:
Eligard 7.5 mg
Germany:
Eligard 7.5 mg
Hungary:
Eligard 7.5 mg
Iceland:
Eligard
Ireland:
Eligard 7.5 mg
Italy:
Eligard
Latvia:
Eligard 7.5 mg
Lithuania:
Eligard 7.5 mg
Luxembourg:
Depo-Eligard 7.5 mg
Netherlands:
Eligard 7.5 mg
Norway:
Eligard
Poland:
Eligard 7.5 mg
Portugal:
Eligard 7.5 mg
Slovakia:
Eligard 7.5 mg
Slovenia:
Eligard 7.5 mg
Sweden:
Eligard
Date of last revision of the leaflet: 10/2024
Information intended for healthcare professionals only:
Before opening, the product should be brought to room temperature by removing it from the refrigerator approximately 30 minutes before use.
First, prepare the patient for administration, and then prepare the solution according to the instructions below. If the solution is not prepared using the appropriate technique, it should not be administered to the patient, as this may result in lack of clinical efficacydue to improper reconstitution of the medicinal product.Step 1
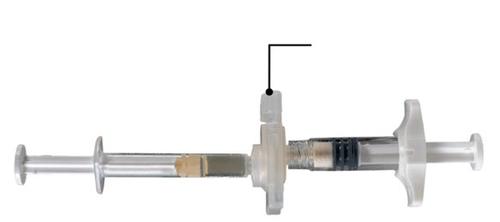
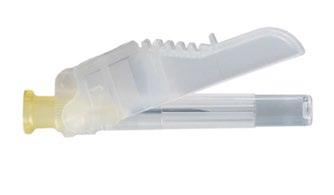
On a clean surface, open the tray by tearing the foil starting from the corners to remove the contents. Remove the pouch with the desiccant. Remove the system of connected syringes (Fig. 1.1) from the tray. Open the package containing the needle with a protective cover (Fig. 1.2) by tearing the paper part of the package.
Note: Syringe A and Syringe B should not be aligned yet.
Fig. 1.1
Tray contents: system of connected syringes
Fig. 1.2
Under the tray: Needle with protective cover and hub
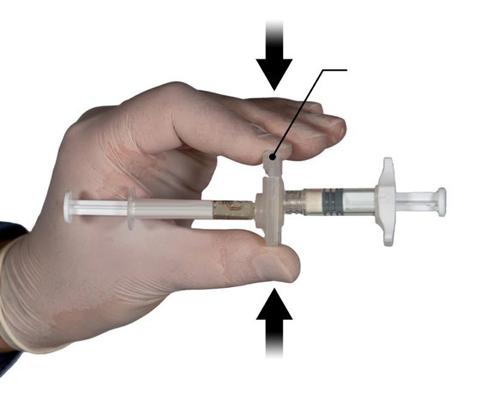
Step 2
With your thumb and index finger, grasp the latch on the connector and press (Fig. 2) until you hear a click. The two syringes will be aligned. Activating the connector does not require any specific positioning of the system of connected syringes. Do not bend the system of connected syringes (as this may cause leakage, as the syringes may become partially unscrewed).
Step 3
Holding the syringes horizontally, move the liquid contents of Syringe A to the leuprolide acetate powder in Syringe B. Mix the product thoroughly by gently moving the contents of both syringes back and forth between the two syringes (one cycle means one push of the Syringe A plunger and one push of the Syringe B plunger) with the syringes in a horizontal position, until a homogeneous, viscous solution is obtained (Fig. 3). Do not bend the system of connected syringes (as this may cause leakage, as the syringes may become partially unscrewed).
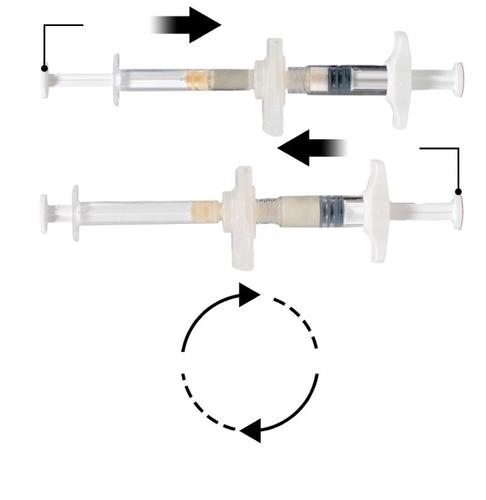
Repeat 60x
After thorough mixing, the viscous solution will be colorless to white or light brown (shades of white to light yellow).
Important: After mixing, proceed immediately to the next step, as the viscosity of the product increases over time. Do not freeze the mixed product.
Note: The product should be mixed according to the instructions; shaking will notensure proper mixing of the product.
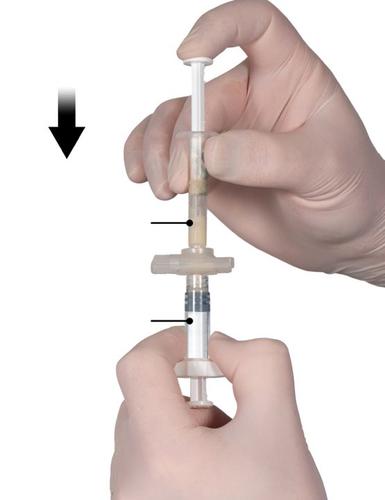
Step 4
After mixing, hold the syringes vertically with Syringe B at the bottom. The syringes should remain properly connected. Move the entire mixed product to Syringe B (short, wide syringe) by pushing the Syringe A plunger and slightly pulling the syringe plunger (Fig. 4)
Fig. 4
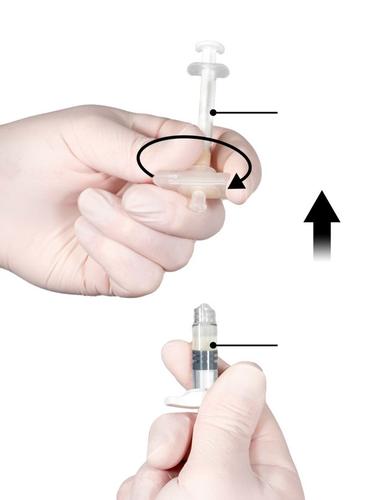
Step 5
While ensuring that the Syringe A plunger is fully depressed, hold the connector and unscrew it from Syringe B. Syringe A will remain connected to the connector (Fig. 5). Ensure that the product does not leak, as the needle, once attached, will not properly secure the syringe.
Note: Some air bubbles may remain in the mixture - this is a normal phenomenon.
Do not remove air bubbles from Syringe B at this stage, as this may cause product loss!
Fig. 5
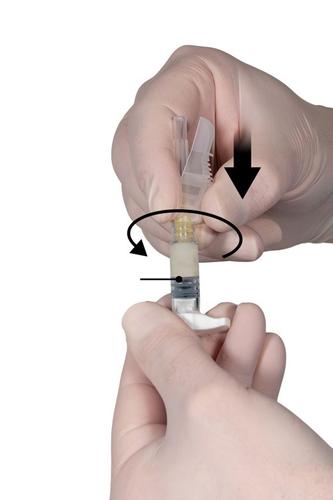
Step 6
- Hold Syringe B vertically and hold the white plunger to prevent product loss.
- Put the needle with the protective cover on Syringe B, holding the syringe, and gently screw the needle about three-quarters of a turn in the direction of the arrow (Fig. 6). Do not overtighten, as this may cause the needle hub to break and result in product leakage during injection .If the needle is tightened too much, the protective cover may also be damaged.
If the needle hub is broken or appears damaged, or if leakage is observed, do not administer the product. Do not replace the damaged needle or exchange it for another, and do not inject the product. Dispose of all unused product in a safe manner.
In the event of needle hub damage, administer a new product.
Step 7
Bend the protective cover away from the needle and remove the needle cover immediately before administering the product (Fig. 7).
Important: Before administration, do not manipulate the needle protective cover mechanism. If the needle hub appears damaged or leakage is observed, do not usethe product. Do not replacethe damaged needle, and do not injectthe product. In the event of needle hub damage, use a new Eligard product.Fig. 7
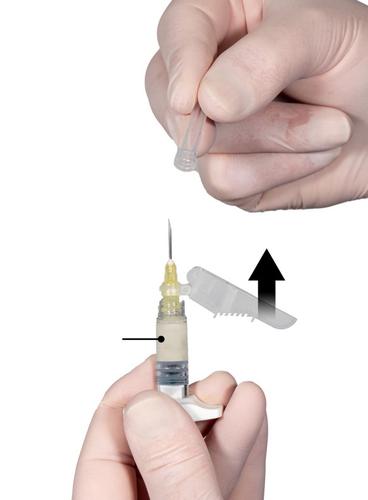
Step 8
Before administering the product, remove all largeair bubbles from Syringe B. Administer the product subcutaneously, keeping the protective cover bent away from the needle.
Administration procedure:
Fig. 8
- Choose an injection site that has not been used recently, on the abdomen, upper buttocks, or other location with sufficient subcutaneous tissue and without discoloration, nodules, or hair.
- Clean the injection site area with an alcohol swab (not provided).
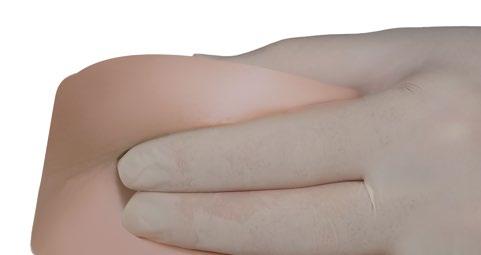
- With your thumb and index finger, grasp and squeeze the skin around the injection site.
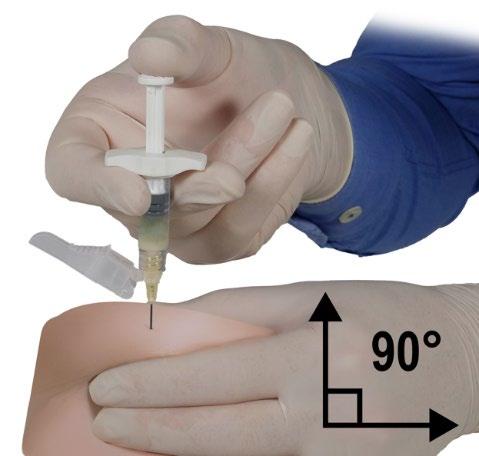
- With your dominant hand, quickly insert the needle at a 90-degree angle to the skin surface. The depth of penetration will depend on the amount and density of subcutaneous tissue and the length of the needle. After inserting the needle, release the skin.
- Inject the product slowly and evenly by pushing the plunger until the syringe is empty. Before removing the needle, ensure that the entire amount of product has been injected from Syringe B.
- Maintaining pressure on the plunger, quickly withdraw the needle at the same 90-degree angle used for insertion.
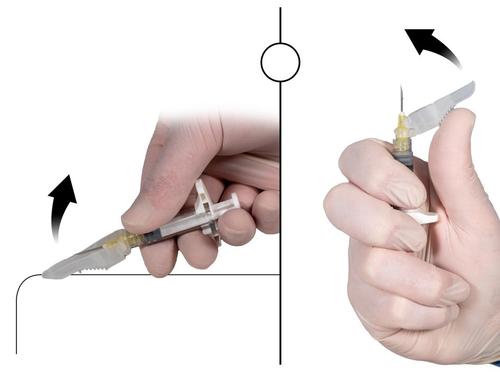
Step 9
After injecting the product, close the needle protective cover using one of the methods below.
1. Closing on a flat surface
Press the protective cover, with the lever facing down, against a flat surface (Fig. 9a) to cover the needle and close the protective cover.
The cover is closed if you hear and feel a click. In the closed position, the needle tip will be completely covered.
2. Closing with the thumb
Place your thumb on the protective cover (Fig. 9b), cover the needle tip, and close the protective cover.
The cover is closed if you hear and feel a click. In the closed position, the needle tip will be completely covered.
Fig. 9a
Closing on a flat surface
Fig. 9b
Closing with the thumb
After closing the protective cover, immediately discard the needle and syringe in a sharps container.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterRecordati Industria Chimica e Farmaceutica S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Eligard 7,5 mgDosage form: Powder, 22.5 mgActive substance: leuprorelinPrescription requiredDosage form: Powder, 45 mgActive substance: leuprorelinPrescription requiredDosage form: Implant, 3.6 mgActive substance: leuprorelinPrescription required
Alternatives to Eligard 7,5 mg in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Eligard 7,5 mg in Spain
Alternative to Eligard 7,5 mg in Ukraine
Online doctors for Eligard 7,5 mg
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Eligard 7,5 mg – subject to medical assessment and local rules.











