

Flixonase Nasule

Ask a doctor about a prescription for Flixonase Nasule

How to use Flixonase Nasule
Leaflet accompanying the packaging: patient information
Flixonase Nasule, 400 g/dose, nasal drops, suspension
Fluticasone propionate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Flixonase Nasule and what is it used for
- 2. Important information before using Flixonase Nasule
- 3. How to use Flixonase Nasule
- 4. Possible side effects
- 5. How to store Flixonase Nasule
- 6. Contents of the packaging and other information
1. What is Flixonase Nasule and what is it used for
The active substance of Flixonase Nasule nasal drops is fluticasone propionate.
It belongs to a group of medicines called steroids or corticosteroids.
The action of steroids is to reduce inflammation:
- reduce swelling and irritation of the nose
- reduce itching, sneezing, feeling of a blocked nose or runny nose (rhinitis).
Flixonase Nasule nasal drops, suspension is used to treat:
- nasal polyps (growths of mucous membrane inside the nose) and related symptoms of reduced nasal patency.
2. Important information before using Flixonase Nasule
When not to use Flixonase Nasule
- if the patient is allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Flixonase Nasule, consult your doctor or pharmacist if the patient:
- has an infection of the upper respiratory tract,
- has adrenal insufficiency,
- is taking ritonavir (see also "Other medicines and Flixonase Nasule").
Consult your doctor if any of the above conditions apply to the patient.
In the case of rare, unilateral nasal polyposis, the diagnosis should be confirmed by a specialist doctor, due to the possibility of another disease.
Patients with nasal polyps require regular medical check-ups.
Avoid contact between the medicine and the eyes and damaged skin.
When using Flixonase Nasule for a long time, the active substance - fluticasone propionate may suppress the natural production of steroid hormones by the adrenal glands.
In the event of adrenal insufficiency, the doctor will consider additional administration of systemic corticosteroids during stress or before a planned surgical procedure.
Due to the possibility of impaired adrenal function, patients who are switching from oral steroid medicines to fluticasone propionate nasal spray should be under special medical care, and their adrenal function should be monitored.
If the patient experiences blurred vision or other vision disturbances that may be caused by cataracts or glaucoma, they should contact their doctor.
There have been reports of growth suppression in children receiving intranasal corticosteroids.
Therefore, the doctor will reduce the dose of the corticosteroid to the smallest dose that provides effective control of symptoms.
Flixonase Nasule and other medicines
Tell your doctor about all medicines the patient is currently taking or has recently taken, as well as any medicines they plan to take, including those available without a prescription.
It is especially important to tell your doctor about any of the following medicines:
- corticosteroids in tablets or injections
- ritonavir or medicines containing cobicistat, used to treat HIV
- ketoconazole or itraconazole, used to treat fungal infections.
The doctor will assess whether Flixonase Nasule can be used with these medicines. Some medicines may enhance the effect of Flixonase Nasule, and the doctor may want to closely monitor the patient's condition (including some HIV medicines: ritonavir, cobicistat).
The doctor will decide whether the patient can use Flixonase Nasule with these medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
The doctor will weigh the benefits and risks to the patient and the child resulting from the use of Flixonase Nasule during pregnancy.
Flixonase Nasule may be used in pregnant women only if, in the doctor's opinion, the benefit to the mother outweighs the potential risk to the fetus.
It is not known whether the active substance of Flixonase Nasule passes into breast milk. If the patient is breastfeeding, they should contact their doctor before using Flixonase Nasule.
Driving and using machines
No effects on the ability to drive or use machines have been observed.
3. How to use Flixonase Nasule
Flixonase Nasule should always be used as directed by the doctor. Do not exceed the recommended dose. If in doubt, consult your doctor.
Flixonase Nasule is intended for use in the nose only in patients over 16 years of age.
Flixonase Nasule should be used daily. The basic condition for the effectiveness of the medicine is its regular use. To achieve full effect, several weeks of treatment are required. Therefore, the medicine should be used even if the patient does not feel an improvement immediately.
When symptoms start to subside, the doctor will recommend reducing the dose of the medicine to the smallest dose that provides effective control of symptoms.
If there is no improvement after 4-6 weeks of treatment, the doctor will consider other treatment methods.
Dosage for patients over 16 years of age
Use the contents of 1 plastic single-dose container once or twice a day.
The plastic container contains a single dose for both nostrils (1 dose - 400 µg).
Administer half of the container contents, approximately 6 drops, once into each nostril.
Instructions for using Flixonase Nasule
- 1. Gently clean the nose.
- 2. Open the aluminum sachet. Use all containers from one sachet before opening the next. The medicine in the plastic containers is suitable for use within 28 days of opening the aluminum sachet.
- 3. Remove one container.
- 4. Shake the container before use to mix its contents thoroughly.
- 5. Remove the top part of the container by twisting it at the point marked "TWIST HERE". The "TWIST HERE" label on the container means "twist here"
- 6. Assume the position as shown in the attached figures A, B, or C:
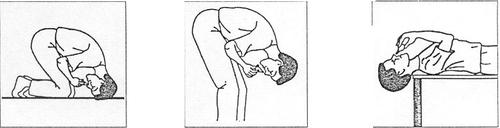
- A. B. C. and administer 6 drops (half of the dose) into each nostril. The container contains a sufficient amount of medicine for both nostrils.
- 7. Remain in the assumed position for at least 1 minute to allow the medicine to spread.
- 8. Discard the container after use.
Dosage for elderly patients
No dose adjustment is necessary for elderly patients.
Dosage for patients under 16 years of age
There is insufficient data on the use of Flixonase Nasule in the treatment of nasal polyps in patients under 16 years of age.
Using a higher dose of Flixonase Nasule than recommended
In the event of using a higher dose of Flixonase Nasule than recommended, consult a doctor or pharmacist for advice.
It is important to take the dose of the medicine as directed by the doctor. Do not take a higher dose of the medicine than recommended by the doctor. Taking smaller or larger doses than recommended may worsen symptoms.
Long-term use of higher-than-recommended doses of Flixonase Nasule may lead to adrenal insufficiency.
Missing a dose of Flixonase Nasule
It is very important to take the recommended dose of the medicine every day, which will ensure the greatest effectiveness of the treatment.
If a dose is missed, take the medicine as soon as possible, and then continue treatment as directed.
Do not take a double dose to make up for a missed dose.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Flixonase Nasule can cause side effects, although not everybody gets them.
The following are side effects that have been observed in patients taking Flixonase Nasule.
Allergic reactions: seek medical help immediately
Allergic reactions to Flixonase Nasule are very rare (may occur in less than 1 in 10,000 patients taking the medicine). In a small number of patients, allergic reactions can develop into a serious condition, even life-threatening, if not treated. Symptoms include:
- Skin rash (hives) or redness,
- Swelling of the face or lips, which can cause difficulty swallowing or breathing,
- Sudden difficulty breathing or worsening of wheezing (bronchospasm),
- Sudden feeling of weakness or dizziness (which can lead to falls or loss of consciousness).
If such symptoms occur, stop using the medicine and contact your doctor immediately, who will prescribe the appropriate treatment.
Very common side effects( may occur in more than 1 in 10 patients):
- Nosebleeds.
Common side effects( may occur in less than 1 in 10 patients):
- Dryness and irritation of the nasal and throat mucosa.
Very rare side effects( may occur in less than 1 in 10,000 patients):
- Allergic reactions,
- Increased intraocular pressure causing vision problems (glaucoma), clouding of the lens of the eye (cataract). Eye symptoms have occurred in patients using Flixonase Nasule for a long time.
- Nasal septum damage.
Side effects with unknown frequency( frequency cannot be estimated from the available data):
- Blurred vision,
- Ulceration of the nasal mucosa.
Tell your doctor as soon as possible if you experience any of these symptoms.
During treatment with Flixonase Nasule, especially in high doses and for a long time, systemic effects may occur due to the suppression of natural steroid hormone production by the adrenal glands.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides
Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309,
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder or its representative.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Flixonase Nasule
Keep the medicine out of the sight and reach of children.
Store in a temperature below 30°C, protected from light.
Do not freeze. Store in an upright position.
The medicine in the plastic containers is suitable for use within 28 days of opening the aluminum sachet.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the specified month.
The batch number of the medicine is stated on the packaging: Lot.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Flixonase Nasule contains
- The active substance of Flixonase Nasule is fluticasone propionate. One dose (one plastic container) of Flixonase Nasule contains 400 µg (micrograms) of fluticasone propionate (micronized).
- The other ingredients are: polysorbate 20, sorbitan laurate, sodium dihydrogen phosphate dihydrate, sodium phosphate anhydrous, sodium chloride, water for injections.
What Flixonase Nasule looks like and what the packaging contains
7 containers - 1 sachet of 7 containers.
28 containers - 4 sachets of 7 containers.
LDPE single-dose containers are connected in a strip, packaged in aluminum sachets, placed in a cardboard box.
Marketing authorization holder: Manufacturer:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
D24 YK11
Ireland
GlaxoSmithKline Trading Services Limited
12 Riverwalk,
Citywest Business Campus
Dublin 24,
Ireland
To obtain more detailed information, contact the representative of the marketing authorization holder:
GSK Services Sp. z o.o.
Rzymowskiego 53
02-697 Warsaw
tel. (22) 576-90-00
GSK logo
Date of last update of the leaflet:January 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxoSmithKline Trading Services Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Flixonase NasuleDosage form: Aerosol, 50 mcg/nasal doseActive substance: fluticasonePrescription requiredDosage form: Aerosol, 50 mcg/dose inh.Active substance: fluticasonePrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: fluticasonePrescription not required
Alternatives to Flixonase Nasule in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flixonase Nasule in Ukraina
Alternative to Flixonase Nasule in Hiszpania
Online doctors for Flixonase Nasule
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flixonase Nasule – subject to medical assessment and local rules.







