
MOMETASONE FUROATE TEVA 50 micrograms NASAL SPRAY SUSPENSION


How to use MOMETASONE FUROATE TEVA 50 micrograms NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for theuser
MometasonefuroateTeva 50 micrograms nasal spray suspension
mometasone furoate
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Mometasone furoate Teva is and what it is used for
- What you need to know before you use Mometasone furoate Teva
- How to use Mometasone furoate Teva
- Possible side effects
- Storing Mometasone furoate Teva
- Contents of the pack and other information
1. What Mometasone furoate Teva is and what it is used for
What is MometasonefuroateTeva?
Mometasone furoate Teva nasal spray contains mometasone furoate, a medicine belonging to a group of medicines called corticosteroids. Mometasone furoate should not be confused with anabolic steroids which are misused by some athletes and given in tablet or injection form. When mometasone furoate is sprayed into the nose, it can help to relieve inflammation (swelling and irritation of the nose), sneezing, itching and blocked or runny nose.
What is MometasonefuroateTeva used for?
Hay fever and perennial rhinitis
Mometasone furoate Teva is used to treat the symptoms of hay fever (also known as seasonal allergic rhinitis) and perennial rhinitis in adults and children aged 3 and above.
Hay fever, which occurs at certain times of the year, is an allergic reaction caused by breathing in pollen from trees, grasses, weeds and also mould spores. Perennial rhinitis occurs throughout the year and the symptoms can be caused by being sensitive to a variety of things including house dust mites, animal hair (or dander), feathers and some foods. Mometasone furoate Teva reduces the inflammation and irritation in your nose and also relieves sneezing, itching and blocked or runny nose caused by hay fever or perennial rhinitis.
Nasal polyps
Mometasone furoate Teva is used to treat nasal polyps in adults aged 18 and above.
Nasal polyps are small growths on the lining of the nose and usually affect both nostrils. Mometasone furoate Teva reduces the inflammation in the nose, causing the polyps to shrink gradually, and relieves the blocked nose that can affect your breathing through your nose.
2. What you need to know before you use Mometasone furoate Teva
Do not use Mometasone furoateTeva:
- if you are allergic to mometasone furoate or any of the other ingredients of this medicine (listed in section 6).
- if you have an untreated nasal infection. Using Mometasone furoate Teva while you have an untreated infection in your nose, such as herpes, may worsen the infection. You should wait until the infection has cleared up before starting to use the nasal spray.
- if you have recently had nasal surgery or had a recent nose injury. You should not use the nasal spray until your nose has healed.
Warnings and precautions
Tell your doctor or pharmacist before you start using Mometasone furoate Teva.
- if you have ever had tuberculosis
- if you have any other infection
- if you are taking other corticosteroids by mouth or by injection
- if you have cystic fibrosis
While you are using Mometasone furoate Teva, tell your doctor:
- if your immune system is not working well (if you are having trouble fighting off infections) and you come into contact with someone who has measles or chickenpox. You should avoid contact with anyone who has these infections.
- if you have a nose or throat infection.
- if you have been using this medicine for several months or longer.
- if you have persistent irritation of your nose or throat.
If nasal corticosteroid sprays are used at high doses for long periods of time, side effects may occur due to the medicine being absorbed into the body.
If your eyes are itchy or irritated, your doctor may advise you to use other treatments at the same time as Mometasone furoate Teva.
Tell your doctor if you have blurred vision or other visual disturbances.
Children
When nasal corticosteroid sprays are used at high doses for long periods of time, they may cause side effects such as slowing down of growth in children.
It is recommended that the height of children using corticosteroid nasal sprays for long periods of time is regularly monitored, and if any changes are observed, they should be reported to the doctor.
Using Mometasone furoateTeva with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription.
If you are taking other corticosteroids for your allergy, by mouth or by injection, your doctor may advise you to stop taking them when you start using Mometasone Teva. When stopping corticosteroids by mouth or injection, some people may get side effects such as muscle or joint pain, weakness, and depression. You may also develop other allergies, such as itchy, watery eyes or itchy skin rashes. If you get any of these effects, you should talk to your doctor.
Some medicines may increase the effects of Mometasone furoate Teva, so your doctor will monitor you closely if you are taking these medicines (including some medicines for treating HIV such as ritonavir, cobicistat).
Pregnancy, breast-feeding and fertility
There is little or no information on the use of Mometasone furoate Teva in pregnant women. It is not known whether mometasone furoate is excreted in breast milk.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
There is no known effect of Mometasone furoate Teva on driving or using machines.
Mometasone furoate Tevacontains benzalkonium chloride
This medicine contains 20 micrograms of benzalkonium chloride in each spray (0.1 ml). Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long-term treatment.
3. How to use Mometasone Teva
Follow exactly the instructions of your doctor or pharmacist. If you are not sure, ask your doctor or pharmacist again. Do not use more than the recommended dose or use the spray more often or for longer than your doctor tells you.
Treatment of hay fever and perennial rhinitis
Use in adults and children over 12 years of age
The recommended dose is two sprays in each nostril once daily
- Once symptoms are under control, your doctor may recommend reducing the dose to one spray in each nostril once daily.
- If you do not start to feel better, you should talk to your doctor and they may increase the dose to the maximum daily dose of four sprays in each nostril once daily.
Use in children aged 3 to 11 years
The recommended dose is one spray in each nostril once daily.
Mometasone furoate Teva may start to relieve symptoms within 12 hours of the first dose; however, it is unlikely that the full effect of the treatment will be seen before the first 2 days. Therefore, you should keep using the spray regularly to achieve the full effect of the treatment.
If you or your child have very severe hay fever, your doctor may advise you to start using Mometasone furoate Teva a few days before the start of the pollen season, as this will help to prevent the symptoms of hay fever. At the end of the pollen season, your hay fever symptoms should improve and treatment may then not be necessary.
Nasal polyps
Use in adults aged 18 and above
The initial recommended dose is two sprays in each nostril once daily.
- If after 5 to 6 weeks the symptoms are not controlled, the dose may be increased to two sprays in each nostril twice daily. Once symptoms are under control, your doctor may reduce the dose.
- If there is no improvement in symptoms after 5-6 weeks of using two sprays in each nostril twice daily, you should talk to your doctor about alternative treatments to Mometasone furoate Teva.
Preparing your nasal spray for use
Mometasone furoate Teva nasal spray has a cap that protects the nozzle and keeps it clean. Remember to remove it before using the spray and replace it after use.
If you are using the spray for the first time, you need to “prime” the bottle by pumping the spray 10 times until a fine spray is produced:
- First, shake the bottle carefully.
- Put your index and middle fingers on either side of the nozzle and your thumb underneath the bottle. Do notpuncture the nasal applicator.
- Do not point the nozzle towards you and then press with your fingers to spray 10 times until a fine spray is produced (Figure 1).
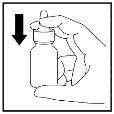
Figure 1
If you have not used your nasal spray for 14 days or more, you need to “prime” the bottle again by pumping the spray 2 times until a fine spray is produced.
How long will your nasal spray last
At the normal dose of two sprays in each nostril once daily for the treatment of hay fever and perennial rhinitis and nasal polyps, this medicine provides enough doses for 15 days (if the bottle contains 60 sprays), 30 days (if the bottle contains 120 sprays) and 35 days (if the bottle contains 140 sprays).
How to use your nasal spray
- Shake the bottle carefully and remove the cap (Figure 2)
Cap Nozzle Bottle |
Figure 2 |
- Gently blow your nose
- Block one nostril and put the nozzle in the other nostril as shown (Figure 3).
Tilt your head slightly forward, keeping the bottle upright.
Direct the nozzle towards the side of the nasal passage, not towards the centre (nasal septum).
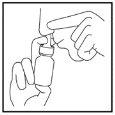
Figure 3
- Start to breathe in slowly through your nose and while you are breathing in, spray the fine spray into your nose by pressing ONCEwith your fingers (Figure 4).
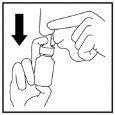
Figure 4
- Breathe out through your mouth. Repeat step 4 to inhale a second spray into the same nostril, if necessary.
- Remove the nozzle from this nostril and breathe out through your mouth.
- Repeat steps 3 to 6 in the other nostril (Figure 5).
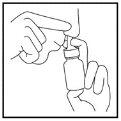
Figure 5
After using the spray, clean the nozzle carefully with a clean tissue or paper handkerchief and replace the cap.
Cleaning your nasal spray
- It is important that you clean your nasal spray regularly, otherwise it may not work properly.
- Remove the cap (Figure 6) and gently pull off the nozzle (Figure 7).
Cap |
Figure 6 | Nozzle |
|
- Wash the cap (Figure 8) and nozzle (Figure 9) with warm water and then rinse with running water.
Cap |
Figure 8 | Nozzle |
|
- Do not try to unblock the nasal applicator by inserting a needle or other pointed object as this may damage the applicator so that you do not receive the correct dose of medicine.
- Put the cap and nozzle to dry in a warm place.
Press the nozzle onto the bottle (Figure 10) and replace the cap (Figure 11).
Central hole Nozzle Pump system |
Figure 10 |
Figure 11 |
- The spray will need to be primed again with 2 sprays when it is used for the first time after cleaning (Figure 12).
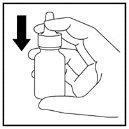
Figure 12
If you use more Mometasone furoate Teva than you should
If you accidentally use more than you should, talk to your doctor.
If you use corticosteroids for a long time or in large amounts, you may rarely get side effects. In children, this can affect growth and development.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, telephone: 91 562 04 20, indicating the medicine and the amount taken. It is recommended to take the packaging and the leaflet of the medicine to the healthcare professional.
If you forget to use Mometasone furoate Teva
If you forget to use your nasal spray at the right time, use it as soon as you remember, and then continue with your normal routine of administration. Do not take a double dose to make up for a forgotten dose.
If you stop using Mometasone furoate Teva
In some patients, Mometasone furoate Teva may start to relieve symptoms 12 hours after the first dose; however, it may take 2 days for the full benefit of the treatment to be seen. It is very important that you use your nasal spray regularly. Do not stop your treatment even if you feel better, unless your doctor tells you to.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Immediate hypersensitivity reactions (allergic) may occur after using this medicine. These reactions can be severe. You should stop treatment with Mometasone furoate Teva and seek immediate medical attention if you experience symptoms such as:
- swollen face, tongue, or pharynx
- difficulty swallowing
- hives
- fatigue or difficulty breathing
If nasal corticosteroid sprays are used at high doses for long periods of time, adverse effects may occur due to the medicine being absorbed into the body.
Other Adverse Effects
Most people do not have any problems after using the nasal spray. However, some people after using Mometasone furoate Teva or other nasal corticosteroid sprays may experience:
Frequent Adverse Effects (may affect up to 1 in 10 people)
- headache
- sneezing and nasal irritation/burning sensation in the nose
- nasal bleeding [very frequent (may affect more than 1 in 10 people) in people with nasal polyps who are administering two sprays of Mometasone furoate Teva in each nostril twice a day.]
- pain in the nose or throat
- ulcers in the nose
- respiratory infection
Unknown Frequency
- increased eye pressure (glaucoma) and/or cataracts that may lead to visual disturbances
- damage to the nasal septum that separates the nostrils
- alterations in taste and smell
- difficulty breathing and/or fatigue
- Blurred vision
Other Adverse Effects in Children and Adolescents
It is expected that the frequency and type of adverse reactions in children will be the same as in adults. Prolonged use of nasal corticosteroids may cause certain adverse effects, such as slowed growth in children. In prolonged treatments with nasal corticosteroids, it is recommended to monitor the child's height from time to time and if changes are observed, inform your doctor.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Mometasone Furoate Teva
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the bottle and on the packaging after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C. Do not freeze.
The spray must be used within 8 weeks of its first administration.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Mometasone Furoate Teva 50 micrograms nasal spray suspension
- The active ingredient is mometasone furoate. Each spray (0.1 ml) contains 50 micrograms of mometasone furoate (as monohydrate).
- The other components are microcrystalline cellulose and sodium carmellose, glycerol, sodium citrate, citric acid monohydrate, polysorbate 80, benzalkonium chloride (see section 2) and water for injectable preparations.
Appearance of the Product and Package Contents
Mometasone Furoate Teva is a nasal spray suspension.
Each bottle contains 60, 120, or 140 sprays.
Bottles containing 60 or 120 sprays are presented in packaging with a single bottle.
Bottles containing 140 sprays are presented in packaging with 1, 2, or 3 bottles.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Teva Pharma, S.L.U.
C/Anabel Segura, 11 Edificio Albatros B, 1ª Planta
28108 Alcobendas, Madrid (Spain)
Manufacturer
Teva Czech Industries s.r.o
Ostravská 305/29,
Komárov 747 70 Opava
Czech Republic
or
Merckle GmbH
Ludwig-Merckle-Str. 3
89143 Blaubeuren, Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria Mometason Ratiopharm 50 mikrogramm/Spruhstoß Nasenspray, Suspension
Germany Mometasonfuroat AbZ 50 Mikrogramm/Sprühstoß Nasenspray, Suspension
Belgium Mometasone Teva 50 microgram per verstuiving, neusspray, suspensie
Denmark Mometasonfuroat Teva
Spain Mometasona furoato Teva 50 micrograms nasal spray suspension
Finland Momesonex 50 mikrog/annos nenäsumute, suspensie
France MOMETASONE TEVA 50 microgrammes/dose, suspension pour pulvérisation nasale.
Hungary Nasotasone 50 mcg szuszpenziós adagolt orrspray
Italy Mometasone TEVA
Lithuania Mometasone Teva 50 mikrogramu/dozeje nosies purškalas (suspensija)
Netherlands Mometasonfuroaat Teva 50 microgram/verstuiving, neusspray, suspensie
Poland Pronasal
Portugal Mometasona Teva, 50 μg/dose, Suspensão para pulverização nasal
Sweden Mometasone Teva 50 mikrogram/dos nässpray, suspension
Date of the last revision of this leaflet: February 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
You can access detailed and updated information about this medicine by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/77974/P_77974.html
- Country of registration
- Average pharmacy price8.99 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MOMETASONE FUROATE TEVA 50 micrograms NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 50 micrograms/sprayActive substance: mometasoneManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 50 microgramsActive substance: mometasoneManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: NASAL PRODUCT, 50 micrograms/actuationActive substance: mometasoneManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for MOMETASONE FUROATE TEVA 50 micrograms NASAL SPRAY SUSPENSION
Discuss questions about MOMETASONE FUROATE TEVA 50 micrograms NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






 Figure 7
Figure 7
 Figure 9
Figure 9








