FLUTICASONA TEVA 50 micrograms NASAL SPRAY SUSPENSION

How to use FLUTICASONA TEVA 50 micrograms NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fluticasona Teva 50 micrograms Nasal Spray Suspension
fluticasone propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Fluticasona Teva and what is it used for
- What you need to know before you use Fluticasona Teva
- How to use Fluticasona Teva
- Possible side effects
- How to store Fluticasona Teva
Contents of the pack and further information
1. What is Fluticasona Teva and what is it used for
Your medicine is called Fluticasona Teva 50 micrograms nasal spray suspension (referred to as “Fluticasona Teva” in this leaflet) and contains 50 micrograms of the active ingredient, fluticasone propionate, in each application. Fluticasone propionate belongs to a group of medicines known as corticosteroids.
Fluticasona Teva has anti-inflammatory properties. When applied to your nose, it will reduce inflammation and irritation. It is used to prevent and treat seasonal allergic rhinitis (e.g. hay fever) and perennial rhinitis (e.g. congestion or runny nose, sneezing and itchiness caused by house dust mites or animals such as cats and dogs). It can be used by adults and children over 4 years old.
2. What you need to know before you use Fluticasona Teva
Do not use Fluticasona Teva
- If you are allergic to fluticasone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Fluticasona Teva:
- If you have had any nasal surgery.
- If you are suffering from, or have recently suffered from, any infection of the nasal passages.
- If you are suffering from, or have recently suffered from, any untreated infection, tuberculosis or eye herpes.
- If you have recently been treated with injected steroids, or have been taking oral steroids for a long time.
- If you are using more than 8 sprays (the maximum recommended daily dose) of Fluticasona Teva per day, as you may need additional steroid treatment in stressful situations.
Fluticasona Teva may reduce your own production of hormones, especially if you use higher doses than recommended for a longer period. In this case, your doctor may need to give you an additional medicine with adrenal hormone for extreme stress, after serious injury or before surgery.
Fluticasona Teva will normally control seasonal allergic rhinitis (allergy) without the need for additional treatment. However, if you are exposed to excessive amounts of pollen, additional treatment may be helpful to control other symptoms such as itchy eyes. Consult your doctor in these cases.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children and adolescents
Fluticasona Teva may have potential systemic effects, which can include growth retardation in children and adolescents, and, more rarely, a variety of psychological or behavioral effects, including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (especially in children).
Using Fluticasona Teva with other medicines
Tell your doctor if you are taking, have recently taken or might take any other medicines, including those obtained without a prescription.
Some medicines may interact with Fluticasona Teva, in particular, consult your doctor or pharmacist if you are taking:
- Any medicine used to treat fungal infections (e.g. ketoconazole)
- Certain medicines may increase the effects of Fluticasona Teva, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
This medicine has no or negligible influence on the ability to drive or use machines.
Fluticasona Teva contains benzalkonium chloride
This medicine contains 40 micrograms of benzalkonium chloride in each released dose.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long periods of treatment.
3. How to use Fluticasona Teva
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is:
Adults (including the elderly) and children over 12 years:
When you start using Fluticasona Teva for the first time, you will normally apply 2 sprays into each nostril once a day, preferably in the morning. Your doctor may increase this dose up to a maximumof 2 sprays into each nostril twice a day.
Once your symptoms are under control, your doctor may reduce your dose to 1 spray into each nostril once a day. If reducing the dose makes your symptoms worse, your dose can be increased back to the original dose.
Children between 4 and 11 years old:
For children aged 4-11 years, the dose is normally 1 spray into each nostril once a day, preferably in the morning. Your doctor may increase this dose up to a maximum of 1 spray into each nostril twice a day.
This medicine is not suitable for children under 4 years old.
Your doctor will prescribe the lowest dose of Fluticasona Teva that effectively controls your symptoms.
This medicine takes a few days to start working. Do not stop taking this medicine unless your doctor tells you to, even if you feel better.
Do not use a higher dose or use Fluticasona Teva more frequently than your doctor has prescribed. It is important not to use this medicine more than your doctor has indicated.
If, despite using this medicine, your eyes itch or water due to hay fever (allergy), tell your doctor. He/She may give you another medicine to treat the symptoms in your eyes.
Before using your nasal spray suspension
Fluticasona Teva has a cap that protects the nozzle from dust - this cap should be removed before using the applicator and replaced after use.
When you are about to use a new pack of Fluticasona Teva, you should check its correct functioning as follows:
- Gently shake the container and remove the dust cap.
- Hold the container upright with your thumb below the bottle and your index and middle fingers on either side of the nozzle. Make sure the nozzle is pointing away from you when you do this.
- Press down with your fingers to release a spray (Figure 1).
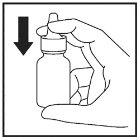 Figure 1
Figure 1
- Repeat steps 2 and 3 about five times - the container is now ready.
If you have not used Fluticasona Teva for 7 days, prepare it by releasing a spray until a fine mist is produced.
If, after trying to prepare the container, it does not work and you think it may be blocked, you can clean it using the following procedure:
Cleaning your nasal spray suspension
- Remove the dust cap (Figure 2) and pull the nozzle upwards to remove it (Figure 3).
Dust cap |
| Nozzle |
|
- Wash the nozzle and dust cap in warm water and soak for a few minutes, then rinse under running water (Figures 4 and 5).
Dust cap |
| Nozzle |
|
- Remove excess moisture by shaking and let the nozzle and dust cap dry in a cool place (not with heat).
- Put the nozzle back on the container (Figure 6).
Central hole Nozzle Spray system |
Figure 6 |
- Check the correct preparation of the container by pressing the spray several times until a fine mist is produced.
- You should clean your nasal spray at least once a week to prevent blockage. Additional cleaning will be needed when the spray appears to be blocked.
- NEVER try to unblock or enlarge the spray hole with a pin or any other sharp object because you may damage the mechanism.
When using your nasal spray suspension
- Shake the container and remove the cap.
- Blow your nose gently.
- Block one nostril by pressing with your finger and put the spray nozzle into the other nostril (Figure 7). Tilt your head slightly forward to keep the container upright.
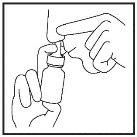 (Figure 7)
(Figure 7)
- Breathe in slowly through your open nostril, at the same time press the nozzle ring downwards with your fingers until a fine spray is released into your nose (Figure 8).
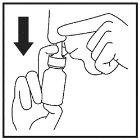 Figure 8
Figure 8
- Breathe out through your mouth. Repeat step 4 to apply a second dose to the same nostril.
- Remove the nozzle from the nostril and breathe out through your mouth.
- Repeat steps 3 to 6 for the other nostril (Figure 9).
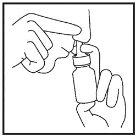 Figure 9
Figure 9
After using your nasal spray suspension
- Gently wipe the nozzle with a clean tissue and replace the cap.
If you use more Fluticasona Teva 50 micrograms nasal spray suspension than you should:
It is important that you take the dose as indicated in this leaflet or as your doctor has told you. You should only use what your doctor recommends; if you use more or less, your symptoms may worsen.
If you accidentally use more Fluticasona Teva than you should, consult your doctor or pharmacist immediately or contact the Toxicology Information Service. Telephone: 91 562 04 20.
Take this leaflet and your Fluticasona Teva to show your doctor.
If you forget to use Fluticasona Teva 50 micrograms nasal spray suspension:
Do not take a double dose to make up for a forgotten dose. If you forget to apply a dose at the right time, use the medicine as soon as you remember. And if it is almost time for the next dose, wait until then and continue as before.
If you stop using Fluticasona Teva 50 micrograms nasal spray suspension:
Your nasal symptoms may start to improve only after using the medicine for a few days, so it is very important that you continue to use the medicine regularly as prescribed, and do not stop using it unless your doctor tells you to, even if you feel better.
If you have any doubts about how to use the medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you are using high doses of Fluticasona Teva, you may need extra steroid treatment in case of extreme stress, or during a hospital stay after a serious injury or before surgery.
Treatment with nasal corticosteroids may affect the body's production of steroids. The likelihood of this occurring increases with the use of high doses for a long period. This may cause children to grow more slowly than others, and therefore, children receiving long-term treatment with nasal corticosteroids will need to have their height checked regularly by their doctor. Your doctor will help prevent this by prescribing the lowest dose of steroids that effectively controls your symptoms.
Some side effects are more serious than others; if you experience any of the following, you should stop using Fluticasona Teva and consult your doctor as soon as possible:
- Severe allergic reactions. Resulting in sudden appearance of a skin rash, swelling (usually of the tongue, face or lips) or difficulty breathing.
- Bronchospasm (narrowing of the airways in the lungs with signs of difficulty breathing and coughing).
- Sudden drowsiness or dizziness (leading to fainting or loss of consciousness)
Other side effects
Very common: may affect up to 1 in 10 people
- Nosebleeds (epistaxis).
Common: may affect up to 1 in 10 people
- Headache.
- Bad breath or unpleasant smell in the nose.
- Dryness and irritation of the throat and nasal passages and discharge.
Very rare: may affect up to 1 in 10,000 people
- During prolonged treatment, glaucoma (increased pressure in the eye) and cataracts (loss of transparency of the lens in the eye) have occurred.
- Perforation of the nasal septum (the wall that divides the nose) and ulceration of the nasal mucous membrane - although this has occurred in patients who had previously undergone nasal surgery.
Not known: cannot be estimated from the available data
- Blurred vision
- Nasal ulceration
Side effects in children and adolescents
Children being treated may grow more slowly than others, and therefore, children receiving long-term treatment with corticosteroids need to have their height checked regularly by their doctor. Your doctor will help prevent this by prescribing the lowest dose of steroids that effectively controls your symptoms.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Monitoring System for Human Use website: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluticasona Teva
Keep out of the sight and reach of children.
Do not use Fluticasona Teva after the expiry date which is stated on the container after the abbreviation EXP. The expiry date is the last day of the month shown.
Do not store above 25°C.
Use within 3 months of first use.
Use the label on the container to note the date of first use.
Medicines should not be disposed of via wastewater or household waste. Return containers and any unused medicines to your pharmacist. If you are unsure, ask your pharmacist how to dispose of containers and any unused medicines. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Fluticasona Teva
The active ingredient is fluticasone propionate.
Each spray contains 50 micrograms of fluticasone propionate.
The other components are glucose, dispersible cellulose, phenylethyl alcohol, benzalkonium chloride (40 micrograms per delivered dose), polysorbate 80, and purified water.
Appearance of the Product and Package Contents
Fluticasona Teva consists of a white and opaque suspension contained in a multidose amber glass bottle with a dosing spray pump to create the spray. Each package contains suspension capable of releasing 60, 120, 150, 240 (2 bottles containing each 120 sprays) or 360 (3 bottles containing each 120 sprays) sprays.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Teva Pharma, S.L.U.
Anabel Segura 11 Edificio B Albatros 1ª planta
28108 Alcobendas, Madrid
Manufacturer:
Teva Czech Industries s.r.o.
Ostravska, 29
Opava, Czech Republic.
or
Teva Operations Poland Sp. z o.o.
ul. Mogilska 80
31-546 Kraków
Poland
This medicinal product is registered in the EEA Member States with the following names
Czech Republic Nasofan, nasní sprej
Germany Flutica-TEVA 50 Mikrogramm Nasenspray, Suspension
Denmark Fluticasonpropionat "Teva" 50 mikrogram/dosis, næsespray, suspension
Spain Fluticasona Teva 50 micrograms suspension for nasal spray
Finland Nasofan 50 mikrog/annos nenäsumute
Hungary Flutirin orrspray
Ireland Nasofan Aqueous 50 microgram Nasal Spray, Suspension
Norway Flutikason Teva
Poland Fanipos, 50 mikrogramów/dawke donosowa aerosol donosa, zawiesina
Portugal Fluticasona Nasofan 50 micrograms Suspension for nasal spray
Slovak Republic Nasofan 50 mikrogramová nosová aerodisperzia aer nas
United Kingdom Nasofan Aqueous 50 microgram Nasal Spray
Date of the last revision of this leaflet:May 2023
Other sources of information
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
You can access detailed and updated information about this medicinal product by scanning the QR code included in the packaging with your mobile phone (smartphone). You can also access this information at the following internet address: https://cima.aemps.es/cima/dochtml/p/67852/P_67852.html
QR Code + URL
- Country of registration
- Average pharmacy price11.18 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUTICASONA TEVA 50 micrograms NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 0.05 mg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: NASAL PRODUCT, 0.05% w/wActive substance: fluticasoneManufacturer: Haleon Spain S.A.Prescription not requiredDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for FLUTICASONA TEVA 50 micrograms NASAL SPRAY SUSPENSION
Discuss questions about FLUTICASONA TEVA 50 micrograms NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




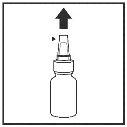 Figure 2
Figure 2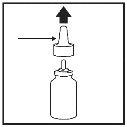 Figure 3
Figure 3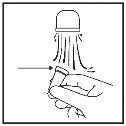 Figure 4
Figure 4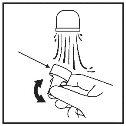 Figure 5
Figure 5