
FLONASE 50 micrograms/spray NASAL SUSPENSION


How to use FLONASE 50 micrograms/spray NASAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Flonase 50 micrograms/spray suspension for nasal spray
Fluticasone Propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
Follow the instructions for administration of the medicine contained in this leaflet or as indicated by your pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or more information, ask your pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 7 days.
Contents of the package leaflet
- What is Flonase and what is it used for
- What you need to know before you start using Flonase
- How to use Flonase
- Possible side effects
- Storage of Flonase
- Contents of the pack and further information
1. What is Flonase and what is it used for
The active substance is fluticasone propionate, a corticosteroid that, when used daily, has anti-inflammatory action. This nasal spray suspension helps control your body's reaction to allergens ("triggers") present in the environment.
Flonase is used in adults aged 18 years and older to relieve symptoms associated with allergic rhinitis caused by pollen and other allergens present in the air, such as pet allergies, dust mites, and mold spores. It relieves symptoms such as sneezing, runny nose, itching or nasal congestion, itchy and watery eyes, for up to 24 hours.
It may take 3 or 4 days to reach the maximum level of protection. Therefore, it is essential to continue regular use to achieve the maximum therapeutic benefit.
You should consult a doctor if your symptoms worsen or do not improve after 7 days.
2. What you need to know before you start using Flonase
Do not use Flonase:
- If you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Flonase:
- If you are using medicines that contain corticosteroids (including creams for eczema, inhalers for asthma, tablets, injections, nasal sprays, or eye drops).
- If you have an infection in the nose or sinuses, if you have a cold, fever, or have recently undergone surgery, injuries, or ulcers in the nose.
If your symptoms do not improve after 7 days, talk to your doctor.
Do not use the medicine continuously for more than 3 months, unless your doctor tells you to.
Contact your doctor if you experience blurred vision or other visual disturbances, which could be caused by diseases such as cataracts or glaucoma.
When nasal corticosteroid sprays are used at high doses for prolonged periods, adverse reactions may occur due to absorption of the medicine by the body. These effects are much less likely than with oral corticosteroids and may vary in individual patients and between different corticosteroid preparations. Potential systemic effects may include Cushing's syndrome, Cushingoid symptoms, adrenal suppression, growth retardation in children and adolescents, and, more rarely, reduced bone mineral density, effects on glucose metabolism, and a range of psychological and behavioral effects, including psychomotor hyperactivity, sleep disturbances, anxiety, depression, or aggression (especially in children).
Treatment with higher doses of nasal corticosteroids than recommended may result in clinically significant adrenal suppression. If there is evidence of use of higher doses than recommended, additional coverage with systemic corticosteroids should be considered during periods of stress or elective surgery.
Using Flonase with other medicines
Please inform your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription, especially:
- Medicines that contain corticosteroids (including creams for eczema, inhalers for asthma, tablets, injections, nasal sprays, or eye drops).
- Ritonavir or cobicistat (a medicine for HIV).
- Ketoconazole (for fungal infections).
Some medicines may increase the effect of Flonase, and your doctor may want to monitor you closely if you are taking these medicines (including some medicines for HIV: ritonavir, cobicistat).
Using Flonase with food and drinks
You can use Flonase at any time of day, with or without food.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. There are no data on the effects of this medicine on fertility.
Driving and using machines
It is unlikely that Flonase will affect your ability to drive or use tools or machines.
Flonase contains benzalkonium chloride
This medicine contains 0.02 mg of benzalkonium chloride per dose of spray solution, equivalent to 0.2 mg of benzalkonium chloride per 1 ml of solution.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for extended periods.
3. How to use Flonase
Follow the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor or pharmacist. If in doubt, ask your doctor or pharmacist.
Adults aged 18 years and older:
The recommended dose is two sprays in each nostril once daily (200 micrograms of fluticasone propionate), ideally in the morning.
Once your symptoms have improved, you can reduce the dose to one spray in each nostril once daily.
If your symptoms are particularly severe, you may need to apply two sprays in each nostril twice daily until your symptoms improve, but this is only for short-term use.
- Do not apply more than 8 sprays (4 sprays per nostril) per day.
- Use the lowest dose possible to control your symptoms.
- If your symptoms do not improve or are not well-controlled after 7 days, consult your doctor or pharmacist.
- Do not use more than the recommended dose.
Use in children and adolescents:
This medicine must not be used in children and adolescents under 18 years of age.
Method of administration
For use in the nose only.
Do not swallow.
Do not spray into the eyes.
Before using a new bottle of Flonase:
The Flonase spray has a cap that protects the nozzle and keeps it clean. Remember to remove it before using the spray (Figure 1).
Before starting a new bottle of Flonase, or if you have not used it for a few days, prime the device (press the spray device several times) to ensure you receive the correct dose.
Hold the bottle with your thumb under and your index and middle fingers on either side of the nozzle. Keeping your thumb fixed, press down with your fingers several times until you get a fine spray. (Figure 2)
When doing this, make sure not to point the nozzle at yourself or others.
If the spray device still does not work, try cleaning it as described in the "Cleaning the spray device" section. Do not attempt to unclog or enlarge the small spray hole with a needle or other sharp object, as this would damage the spray mechanism.
Figure 1
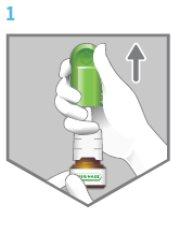
Figure 2

Using Flonase:
- Blow your nose gently.
- Shake the bottle well and remove the protective cap.
- If it is a new bottle of Flonase, or if you have not used the spray device for a few days, you will need to prime the device. Press the spray device down several times until you get a fine spray.
- Hold the bottle as shown in Figure 3. Place the nozzle in one nostril and block the other.
- Lean your head forward slightly and keep the bottle straight, as shown in Figure 4. Aim slightly away from the center of your nose.
- Start breathing in slowly through your nose. As you breathe in, press firmly on the neck of the spray device with your fingers to spray the medicine inside your nose (Figure 4).
- Breathe out through your mouth. Repeat step 4 to apply a second spray in the same nostril.
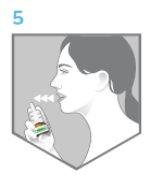
- Remove the nozzle from the nostril you have just sprayed and breathe out through your mouth (Figure 5).
- Repeat steps 4 to 6 in the other nostril.
- After using the spray device, clean the nozzle carefully with a clean tissue and replace the protective cap.
Figure 3
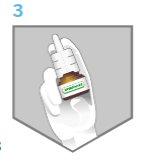
Figure 4
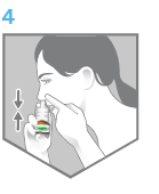
Figure 5
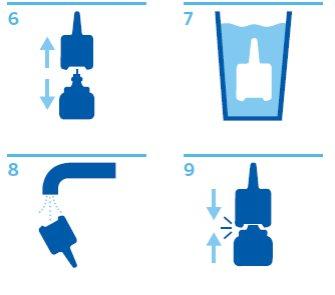
Cleaning the spray device:
- Remove the protective cap and nozzle (Figure 6).
- Soak the nozzle and protective cap in warm water for a few minutes (Figure 7) and then rinse under the tap (Figure 8).
- Shake off excess water and let it dry in a warm place - not too hot!
- Put the nozzle back on the spray device (Figure 9).
- If necessary, press the spray device down several times until you get a fine spray. Replace the protective cap.
- Do not attempt to unclog or enlarge the small spray hole with a needle or other sharp object, as this would damage the spray mechanism.
Figures 6, 7, 8, 9
It may take 3 or 4 days to reach the maximum level of protection provided by Flonase. To prevent symptoms, start using Flonase before exposure to your usual "triggers".
Do not use Flonase for longer than you are exposed to your usual "triggers" (e.g., pollen).
If you use more Flonase than you should
Talk to your doctor if you use more Flonase than recommended.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Flonase
If you forget a dose, simply use the next dose when it is due. Do not use a double dose to make up for the forgotten dose.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
When nasal corticosteroid sprays are used at high doses for prolonged periods, side effects may occur due to absorption of the medicine by the body.
Some side effects can be serious.
Side effects of very rare but serious frequency, seek medical help immediately.
- Signs of allergic reaction, such as skin rash, swelling of the mouth or face, or difficulty breathing.
- Changes in vision that develop after starting to use this product. Rare cases of eye problems, such as cataracts or glaucoma, have been reported in people who have used large amounts of corticosteroids over several years.
- Small holes (perforations) in the nasal septum.
- Eye problems, such as pain.
Side effects of unknown frequency
The frequency of these side effects is not known
- Blurred vision.
- Sores in the nose.
If you develop any of these symptoms, stop using the medicine and seek medical help immediately.
Other side effects
Very common side effects
Affect more than 1 in 10 people
- Occasional nasal bleeding
Common side effects
May affect up to 1 in 10 people
- Sneezing after using the spray. This stops soon.
- Unpleasant smell or taste.
- Dryness or irritation of the nose or throat.
- Headache.
Rare side effects
May affect up to 1 in 10,000 people
- Nasal problems such as pain and/or persistent bleeding.
If you experience any of these symptoms, stop using the medicine and seek medical help.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Flonase
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the bottle after "EXP". The expiry date is the last day of the month indicated.
Do not store above 30°C.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Flonase
- The active substance is fluticasone propionate. Each spray delivers 100 mg of suspension containing 50 micrograms of fluticasone propionate as the delivered dose.
- The other ingredients (excipients) are glucose (anhydrous), microcrystalline cellulose, sodium carmellose, phenylethanol, benzalkonium chloride, polysorbate 80, and purified water.
Appearance of the product and pack contents
Flonase is a white, opaque aqueous nasal spray suspension
The amber glass bottle is equipped with a spray device, nasal applicator, and protective cap. The bottle contains 60 sprays, with a total content of not less than 7.0 g, or 120 sprays, with a total content of not less than 14.0 g.
Marketing authorisation holder and manufacturer
Marketing authorisation holder:
Haleon Spain, S.A.
Paseo de la Castellana, 259D, 32nd floor - 28046 - Madrid - Spain.
Manufacturer:
Glaxo Wellcome S.A., 09400, Aranda de Duero, Burgos, Spain
or
Haleon Germany GmbH
Barthstrasse 4, 80339 Munich, Germany
This medicine is authorised in the Member States of the European Economic Area under the following names:
Belgium Otrivine Anti-Allergie 50 micrograms/dose, suspension for nasal spray
Estonia Flixonase, 50 micrograms/spray, nasal spray suspension
Hungary Flixonase Allergia 50 micrograms/dose, nasal spray suspension
Ireland Fluticasone propionate 50 micrograms/actuation, nasal spray, suspension
Latvia Flixonase 50 micrograms/actuation, nasal spray, suspension
Luxembourg Otrivine Anti-Allergie 50 micrograms/dose, suspension for nasal spray
Netherlands Flixonase GSK ConsHealth 50 micrograms/dose, nasal spray, suspension
Norway Otrason 50 micrograms/dose, nasal spray, suspension
Poland OtriAllergy Control 50 micrograms/dose, nasal spray, suspension
Portugal Vibrocil Anti-Alergias 50 μg/dose, suspension for nasal spray
Romania Flonase 50 micrograms/spray, nasal spray, suspension
Spain Flonase 50 micrograms/spray, suspension for nasal spray
United Kingdom (Northern Ireland) Fluticasone propionate 50 micrograms/actuation, nasal spray, suspension
Date of last revision of this leaflet:July 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLONASE 50 micrograms/spray NASAL SUSPENSIONDosage form: NASAL PRODUCT, 0.05 mg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: NASAL PRODUCT, 100 MG CONTAINS 50 MCG OF FLUTICASONE PROPIONATEActive substance: fluticasoneManufacturer: Teva Pharma S.L.U.Prescription requiredDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for FLONASE 50 micrograms/spray NASAL SUSPENSION
Discuss questions about FLONASE 50 micrograms/spray NASAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











