FLIXONASE 50 micrograms/spray, NASAL SPRAY SUSPENSION

How to use FLIXONASE 50 micrograms/spray, NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Flixonase 50 micrograms/spray, nasal spray suspension
Fluticasone propionate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet (see section 4).
Contents of the pack:
- What is Flixonase and what is it used for
- What you need to know before you use Flixonase
- How to use Flixonase
- Possible side effects
- Storing Flixonase
- Contents of the pack and further information
1. What is Flixonase and what is it used for
Flixonase is a medicine belonging to the family of corticosteroids and is recommended to prevent and treat the symptoms of seasonal and perennial allergic rhinitis, which can include: sneezing, nasal itching and discharge, blockage, congestion, poor nasal breathing, pain and pressure around the nose and eyes (sinuses), in adults and children over 4 years of age.
Corticosteroids prevent inflammation and reduce the symptoms of the allergic reaction when administered directly into the nasal cavity.
Rhinitis is caused by small particles that are inhaled through the nose, which are not recognized as the body's own and are called allergens. The body tries to eliminate these substances, which can cause sneezing, runny eyes, and itchy eyes, as well as nasal discharge that can block the nose. This can occur at the beginning of summer due to the inhalation of pollen from grass or trees and is called seasonal allergic rhinitis (hay fever). Some people have these problems throughout the year, which is then called perennial rhinitis, often due to dust mites or pet hair (cats, dogs).
2. What you need to know before you use Flixonase
Do not use Flixonase
If you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist:
Consult your doctor if you experience blurred vision or other visual disturbances, which can be caused by cataracts or glaucoma.
Using Flixonase with other medicines
Tell your doctor or pharmacist that you are using, have recently used, or may need to use any other medicine, including those obtained without a prescription.
Consult your doctor before using fluticasone propionate nasally if you are taking:
- medicines containing ritonavir or cobicistat (for the treatment of HIV or AIDS). Some medicines may increase the effects of Flixonase, so your doctor will monitor you closely if you are taking these medicines.
- oral medicines for the treatment of fungal infections (ketoconazole).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
No studies have described any effect of Flixonase on driving or operating machinery. Check if Flixonase affects you before driving or operating heavy machinery.
Flixonase contains benzalkonium chloride
This medicine contains 0.02 mg of benzalkonium chloride per spray.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for long periods of treatment.
Use in athletes
Athletes are informed that this medicine contains a component that may result in a positive doping test result.
3. How to use Flixonase
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. Do not exceed the recommended dose. If in doubt, consult your doctor or pharmacist again.
Adults and children over 12 years
The recommended dose is 2 sprays in each nostril once a day, preferably in the morning. Your doctor may advise you to increase the dose to a maximum of 2 sprays in each nostril twice a day (morning and evening).
Use in children from 4 to 11 years
The normal dose is 1 spray in each nostril once a day, preferably in the morning. Your doctor may advise you to increase the dose to 1 spray in each nostril twice a day (morning and evening).
This medicine starts to work after a few days. It is very important that you use it regularly. Do not stop treatment even if you feel better, unless your doctor tells you to do so.
Flixonase should only be used in the nose.
How to use the nasal spray
Nasal spray appearance
The nasal spray has a cap that protects the nasal applicator from dust and keeps it clean. Remember to remove the cap before using the spray. A new spray (or one that has not been used for a few days) may not work the first time, so you will need to follow the instructions for "Preparing the nasal spray".
Preparing the nasal spray
You must prepare the nasal spray:
- Before using it for the first time
- If you have not used it for a few days
- If you have just cleaned it following the instructions in the "Cleaning the nasal spray" section
Preparing the nasal spray helps ensure that you always receive the full dose of medicine. Follow these steps:
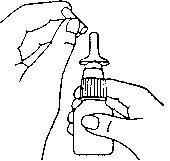
- Shake the nasal spray and remove the cap (see drawing 1).
- Hold the spray in an upright position and point the nasal applicator away from you.
- Place your index and middle fingers on either side of the nasal applicator and your thumb under the bottle (see drawing 2).
DRAWING 1DRAWING 2
|
|
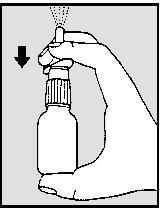
- Keep your thumb and press firmly downwards with your fingers to release a fine spray mist into the air (see drawing 2).
- The nasal spray is now ready for use.
- If you think the nasal applicator may be blocked, do not use a pin or any other sharp object to unblock it.
- Try to clean the spray following the instructions in "Cleaning the nasal spray".
Using the nasal spray
- Shake the nasal spray and remove the cap.
- Blow your nose to clear your nostrils.
- Close one nostril with your finger and carefully place the nasal applicator in the other nostril.
nasal. Tilt your head slightly forward and keep the nasal spray in an upright position (see drawings 3a and 3b).
DRAWING 3aDRAWING 3b
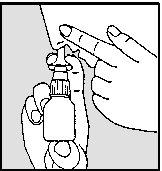
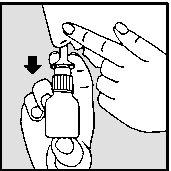
- Press firmly downwards with your fingers while inhaling through your nose (see drawings 3a and 3b).
- Remove the applicator and exhale through your mouth.
- Repeat steps 3 to 4 in the other nostril.
- After using the spray, carefully clean the nasal applicator with a clean tissue.
- Replace the cap.
Cleaning the nasal spray
The nasal spray should be cleaned at least once a week to prevent blockage of the nasal applicator. Follow these steps:
- Remove the protective cap.
- Soak the nasal applicator and cap in warm water for a few minutes.
- Rinse under the tap.
- Remove excess water by shaking and let it dry in a warm place.
- Replace the nasal applicator on the spray.
- Prepare the nasal spray following the instructions in "Preparing the nasal spray" so that it is ready for use.
If you use more Flixonase than you should
If you have used more Flixonase than you should, consult your doctor or pharmacist or the Toxicology Information Service, phone: 91 562 04 20.
It is important that you take your dose as indicated by your doctor. You should only use what your doctor recommends, using more or less dose may worsen your symptoms.
If you forget to use Flixonase
Do not take a double dose to make up for forgotten doses. If you forget a dose, wait until the next dose is due.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some people may be allergic to this medicine, although this is rare.
The systemic effects of nasal corticosteroids may occur, especially when using high doses for prolonged periods.
The following side effects are associated with Flixonase:
Very common side effects(may affect more than 1 in 10 people)
- Nosebleeds (spontaneous bleeding from the nose).
Common side effects(may affect up to 1 in 10 people)
- Headache, unpleasant taste or smell.
- Nasal dryness, nasal irritation, throat dryness, and throat irritation.
Very rare side effects(may affect up to 1 in 10,000 people)
- Hypersensitivity reactions, anaphylaxis/anaphylactoid reactions (generalized allergic reactions), bronchospasm (spasm in the bronchi that prevents air from entering the lungs), skin rash, swelling (edema) in the face or tongue.
- Glaucoma (increased intraocular pressure), cataracts.
- Nasal septum perforation.
Side effects with frequency not known (cannot be estimated from the available data)
- Blurred vision
- Nasal ulcers
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines and Health Products Agency (AEMPS) https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Flixonase
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Do not use this medicine after the expiry date stated on the packaging. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Flixonase
The active substance is fluticasone propionate. Each spray delivers 50 micrograms of fluticasone propionate.
The other ingredients are anhydrous glucose, microcrystalline cellulose, and sodium carmellose, phenylethyl alcohol, benzalkonium chloride, polysorbate 80, diluted hydrochloric acid (for pH adjustment), and purified water.
Appearance of the product and pack contents
The medicine is a nasal spray suspension contained in a polypropylene bottle.
Each bottle of Flixonase contains approximately 120 sprays.
Marketing authorization holder and manufacturer
Marketing authorization holder:
GlaxoSmithKline, S.A.
P.T.M. C/ Severo Ochoa, 2
28760 Tres Cantos (Madrid)
Tel: +34 900 202 700
Manufacturer:
Glaxo Wellcome, S.A.
Avenida de Extremadura, 3
09400 Aranda de Duero (Burgos)
Date of last revision of this leaflet:February 2018.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price11.18 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLIXONASE 50 micrograms/spray, NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 0.05% w/wActive substance: fluticasoneManufacturer: Haleon Spain S.A.Prescription not requiredDosage form: NASAL PRODUCT, 100 MG CONTAINS 50 MCG OF FLUTICASONE PROPIONATEActive substance: fluticasoneManufacturer: Teva Pharma S.L.U.Prescription requiredDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription required
Online doctors for FLIXONASE 50 micrograms/spray, NASAL SPRAY SUSPENSION
Discuss questions about FLIXONASE 50 micrograms/spray, NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions