
Flixonase
Ask a doctor about a prescription for Flixonase

How to use Flixonase
Leaflet accompanying the packaging: patient information
Flixonase, 50 μg/dose, nasal spray, suspension
(Fluticasone propionate)
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Flixonase nasal spray and what is it used for
- 2. Important information before using Flixonase nasal spray
- 3. How to use Flixonase nasal spray
- 4. Possible side effects
- 5. How to store Flixonase nasal spray
- 6. Contents of the packaging and other information
1. What is Flixonase nasal spray and what is it used for
The active substance of Flixonase nasal spray is fluticasone propionate.
It belongs to a group of medicines called steroids or corticosteroids.
The action of steroids is to reduce inflammation:
- reducing swelling and irritation of the nose,
- reducing itching, sneezing, feeling of a blocked nose or runny nose (rhinitis).
Flixonase nasal spray, suspension is used to prevent and treat:
- seasonal allergic rhinitis (including hay fever),
- perennial allergic rhinitis.
Flixonase nasal spray, suspension can also be used for symptomatic treatment of nasal polyps (growths of the mucous membrane inside the nose).
2. Important information before using Flixonase nasal spray
When not to use Flixonase nasal spray
- if the patient is allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Flixonase nasal spray, the patient should discuss it with their doctor or pharmacist if they:
- have an infection of the upper respiratory tract,
- have adrenal insufficiency,
- are taking the medicine ritonavir (see also section "Flixonase nasal spray and other medicines").
The patient should consult their doctor to see if these circumstances apply to them.
In the case of using Flixonase nasal spray for a long time, the active substance - fluticasone propionate may suppress the natural production of steroid hormones by the adrenal glands. In the case of adrenal insufficiency, the doctor will consider additional administration of systemic corticosteroids during stress or before a planned surgical procedure.
Due to the possibility of impaired adrenal function, patients who are switching from oral steroid medicines to fluticasone propionate nasal spray should be under special medical supervision, and their adrenal function should be monitored.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
During long-term nasal corticosteroid treatment, the doctor will recommend regular growth measurements in children.
Flixonase nasal spray and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take, including those that are available without a prescription.
It is especially important to tell the doctor about any of the following medicines that the patient is currently taking or has recently taken:
- corticosteroids in tablets or injections
- ritonavir or medicines containing cobicistat, used to treat HIV
- ketoconazole or itraconazole, used to treat fungal infections.
The doctor will assess whether Flixonase can be used with these medicines. Some medicines may enhance the effect of Flixonase, and the doctor may want to closely monitor the patient's condition when taking such medicines (including some HIV medicines: ritonavir, cobicistat).
The doctor will decide whether the patient can use Flixonase nasal spray with these medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant or plans to have a child, they should consult their doctor before using this medicine. The doctor will weigh the benefits and risks to the patient and the child resulting from the use of Flixonase nasal spray during pregnancy.
Flixonase nasal spray can be used in pregnant women only in cases where the doctor believes that the benefit to the mother outweighs the potential risk to the fetus.
It is not known whether the active substance of Flixonase passes into breast milk. If the patient is breastfeeding, they must contact their doctor before using Flixonase.
Driving and using machines
No effect of the medicine on the ability to drive or use machines has been observed.
The medicine contains benzalkonium chloride
This medicine contains 0.02 mg of benzalkonium chloride per dose. Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
Benzalkonium chloride may also cause wheezing or breathing difficulties (bronchospasm), especially in patients with asthma.
3. How to use Flixonase nasal spray
This medicine should always be used in accordance with the doctor's recommendations. Do not exceed the recommended dose. In case of doubts, consult a doctor.
Flixonase nasal spray is intended for use in the nose only.
Avoid contact of the medicine with the eyes.
The basic condition for the effectiveness of the medicine is its regular use. The full therapeutic effect in the case of treating allergic rhinitis occurs after 3-4 days of regular use. The full therapeutic effect in the case of treating nasal polyps occurs after several weeks of regular use.
Flixonase should be taken for as long as the doctor recommends. Consult a doctor before stopping the use of Flixonase.
Prevention and treatment of allergic, seasonal rhinitis and perennial rhinitis
Dosage for adults and children over 12 years old
Two doses of the spray into each nostril once a day, preferably in the morning.
In some cases, it is recommended to administer two doses into each nostril twice a day. Do not administer more than four doses into each nostril per day.
The doctor will recommend the smallest dose that provides effective control of symptoms.
Dosage for elderly patients
The same doses are used as for adults.
Dosage for children from 4 to 11 years old
One dose into each nostril once a day, preferably in the morning.
In some cases, it is recommended to administer one dose into each nostril twice a day. Do not administer more than two doses into each nostril per day.
The doctor will recommend the smallest dose that provides effective control of symptoms.
Symptomatic treatment of nasal polyps
Dosage for adults
Initially, two doses of the spray into each nostril twice a day (morning and evening) for 1 to 2 months until symptoms are controlled. Do not administer more than four doses into each nostril per day.
Then, two doses of the spray into each nostril once a day for three months.
Dosage for elderly patients
The same dosage as for adults.
Dosage for children and adolescents
Flixonase should not be used in children and adolescents for the treatment of nasal polyps.
Instructions for using Flixonase nasal spray
Before use
- 1. Gently shake the bottle with the medicine and remove the protective cap.
- 2. Hold the bottle as shown in the picture.
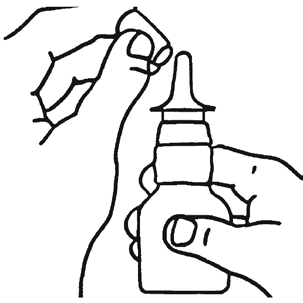
- 3. If Flixonase is used for the first time or after a break of about a week, check if the dispenser works. From a distance, press the dispenser several times. The medicine should appear as a mist.
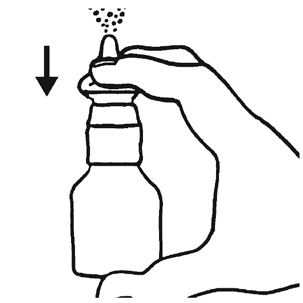
Using the medicine
- 4. Gently blow your nose.
- 5. Block one nostril with your finger, as shown in the picture, and insert the dispenser into the other.
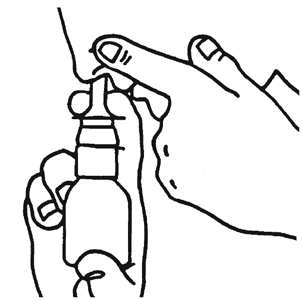
Lean your head slightly forward and hold the dispenser vertically.
- 6. Breathe in through your nose and WHILE INHALING, press the dispenser once with your fingers, releasing the medicine.
- 7. Exhale through your mouth.
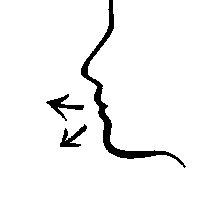
Repeat the actions described in points 6 and 7 to administer the second dose of the medicine.
- 8. Then, switch the bottle to the other hand and repeat the actions to administer the medicine to the other nostril.
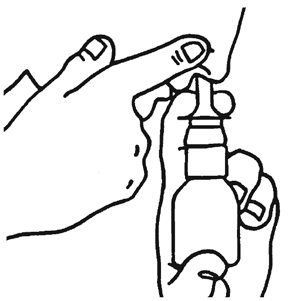
After use
- 9. Wipe the dispenser with a clean cloth or handkerchief and put on the protective cap.
Cleaning
- 1. Gently remove the dispenser. Wash it in warm water.
- 2. Shake off the remaining water and let it dry. Do not dry in high temperature.
- 3. Gently put the dispenser back on the bottle with the medicine. Put on the protective cap.
- 4. If the dispenser becomes clogged, it can be removed and soaked in warm water for a few minutes, then rinsed under cold water. Dry and put back on the bottle. Do not restore the dispenser's patency with a pin or other sharp object.
Using a higher dose of Flixonase nasal spray than recommended
In the event of using a higher dose of Flixonase nasal spray than recommended, consult a doctor or pharmacist for advice.
It is important to take the dose of the medicine as recommended by the doctor. Do not take a higher dose of the medicine than recommended by the doctor. Using doses that are smaller or larger than recommended may cause the symptoms to worsen.
Long-term use of higher-than-recommended doses of Flixonase nasal spray may lead to adrenal insufficiency.
Missing a dose of Flixonase nasal spray
It is very important to take the recommended dose of the medicine every day, which will ensure the greatest effectiveness of the treatment.
In the event of missing a dose, take the medicine as soon as possible, and then continue the treatment as recommended.
Do not take a double dose of the medicine to make up for the missed dose.
Stopping the use of Flixonase nasal spray
Flixonase should be taken for as long as the doctor recommends. Consult a doctor before stopping the use of Flixonase.
In case of any further doubts related to the use of the medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed in patients taking Flixonase nasal spray.
Allergic reactions: seek medical help immediately
Allergic reactions to Flixonase nasal spray are very rare (may occur in less than 1 in 10,000 patients taking the medicine). In a small number of patients, allergic reactions can develop into a serious condition, even life-threatening, if left untreated.
The symptoms include:
- skin rash (hives) or redness,
- swelling of the face or tongue, which can cause difficulty swallowing or breathing,
- sudden difficulty breathing or sudden worsening of wheezing (bronchospasm),
- sudden feeling of weakness or dizziness (which can lead to a fall or loss of consciousness).
In the event of such symptoms, stop using the medicine and seek medical help immediately.
Very common side effects( may occur in more than 1 in 10 patients):
- nasal bleeding.
Common side effects( may occur in 1 to 10 in 100 patients):
- headache, unpleasant taste, unpleasant smell;
- dryness and irritation of the nasal and throat mucous membranes.
Very rare side effects( may occur in less than 1 in 10,000 patients):
- allergic reactions;
- increased intraocular pressure causing vision problems (glaucoma), clouding of the lens of the eye (cataract);
- damage to the nasal septum.
Tell your doctor as soon as possible if you experience any of these symptoms.
Side effects with unknown frequency( frequency cannot be estimated from the available data):
- blurred vision;
- ulceration of the nasal mucous membrane.
During the use of Flixonase nasal spray, especially in high doses and for a long time, systemic symptoms may occur due to the suppression of natural steroid hormone production by the adrenal glands.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Tel: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Flixonase nasal spray
Keep the medicine out of the sight and reach of children.
Store in a temperature below 30°C.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
The batch number of the medicine is stated on the packaging after Lot.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Flixonase nasal spray contains
- The active substance of the medicine is fluticasone propionate. One dose of Flixonase nasal spray contains 50 μg (micrograms) of fluticasone propionate (micronized).
- The other ingredients are: anhydrous dextrose, microcrystalline cellulose and carboxymethylcellulose, phenylethyl alcohol, benzalkonium chloride (50% solution), polysorbate 80, hydrochloric acid diluted to pH 6.3 - 6.5, purified water.
What Flixonase looks like and what the packaging contains
A 20 ml glass bottle with a dispenser connected to a VP3 type spray pump (which consists of elements made of, among others, polypropylene, low-density polyethylene, and ethylene-vinyl acetate) or an SP270+ type spray pump (which consists of elements made of, among others, polypropylene, low-density polyethylene, polyethylene, and ethylene-vinyl acetate) and a protective cap, containing 120 doses, in a cardboard box.
Marketing authorization holder: Manufacturer:
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
D24 YK11
Ireland
Glaxo Wellcome S.A.
Avenida de Extremadura 3
09400 Aranda de Duero
Burgos
Spain
To obtain more detailed information, contact the representative of the marketing authorization holder:
GSK Services Sp. z o.o.
ul. Rzymowskiego 53
02-697 Warsaw
tel. (22) 576-90-00
GSK logo
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGlaxo Wellcome S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FlixonaseDosage form: Aerosol, 50 mcg/nasal doseActive substance: fluticasonePrescription requiredDosage form: Drops, 400 mcg/nasal doseActive substance: fluticasonePrescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: fluticasonePrescription not required
Alternatives to Flixonase in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flixonase in Ukraina
Alternative to Flixonase in Hiszpania
Online doctors for Flixonase
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flixonase – subject to medical assessment and local rules.








