NASACORT 55 micrograms/dose, NASAL SPRAY SUSPENSION

How to use NASACORT 55 micrograms/dose, NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Patient Information Leaflet
Nasacort 55 micrograms/dose, nasal spray suspension
Triamcinolone acetonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Nasacort and what is it used for
- What you need to know before you use Nasacort
- How to use Nasacort
- Possible side effects
5 Storage of Nasacort
- Contents of the pack and other information
1. What is Nasacort and what is it used for
Nasacort contains a medicine called triamcinolone acetonide. This belongs to a group of medicines called corticosteroids, which means it is a type of steroid. It is given as a nasal spray to treat nasal symptoms of allergic rhinitis in adults and children over 2 years old.
The nasal symptoms of allergy include sneezing, itching, and blocked, congested or runny nose. They can be caused by:
- Pet hair or dust mites. This type of allergy can occur at any time of the year and is called “perennial allergic rhinitis”.
- Pollen. This type of allergy, such as hay fever, can be caused by different pollens at different times of the year. It is called “seasonal allergic rhinitis”.
This medicine will only work if you use it regularly. It may take 3 to 4 days before you notice an improvement in your symptoms.
2. What you need to know before you use Nasacort
Do not use Nasacort:
- if you are allergic to triamcinolone acetonide or any of the other ingredients of this medicine (listed in section 6).
The symptoms of an allergic reaction to Nasacort include: rash (hives), itching, problems swallowing or breathing, swelling of the lips, face, throat or tongue.
Warnings and precautions
Tell your doctor or pharmacist before you start using Nasacort:
- if you have any infection in your nose or throat that is not being treated. If you develop a fungal infection while using Nasacort, stop using the spray until the infection has been treated.
- if you have recently had an operation on your nose, or have had injuries or ulcers in your nose.
- if you are being transferred from steroid injections or tablets to Nasacort nasal spray.
- if you have had glaucoma or cataracts.
Get medical help if you have blurred vision or other changes in your vision.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using this medicine.
Children (under 2 years)
This medicine is not recommended for use in children under 2 years old.
Surgery or stressful situations
Your doctor may advise you to take a higher dose of this medicine than usual for medical reasons. If you are going to have an operation or you are not feeling well, tell your doctor. This is because higher than usual doses of this medicine may reduce your body's ability to cope with stress. If this happens, your doctor may decide that you need extra treatment with another medicine to help you.
Using Nasacort with other medicines
Tell your doctor if you are taking or have recently taken any other medicines, including those obtained without a prescription.
Some medicines may increase the effects of Nasacort, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Nasacort is not likely to affect your ability to drive or use machines.
Nasacort contains benzalkonium chloride
This medicine contains 15 micrograms of benzalkonium chloride in each spray.
Benzalkonium chloride may cause irritation or inflammation inside the nose, especially when used for a long time.
Systemic effects
When corticosteroids, including triamcinolone acetonide, are used for long periods of time or in high doses for long periods, side effects can occur due to the medicine being absorbed into the body (e.g. the normal production of steroid hormones may be affected). These side effects are much less likely with nasal steroid sprays than with steroid tablets or injections. Talk to your doctor if you experience any side effects.
3. How to use Nasacort
NASACORT should only be used in the nose
Follow exactly the instructions given to you by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist again. This medicine will only work if you use it regularly. It may take 3 to 4 days before you notice an improvement in your symptoms.
How much Nasacort to use
Adults and children (over 12 years)
- The usual starting dose is 2 sprays in each nostril once a day.
- Once your allergy symptoms are under control, you may be able to reduce your dose to 1 spray in each nostril once a day.
Children (6 to 12 years)
- The usual dose is 1 spray in each nostril once a day.
- If symptoms do not improve, your doctor may increase the dose to 2 sprays in each nostril once a day.
- After this, the dose can be reduced to 1 spray in each nostril once a day.
Children (2 to 5 years)
- The usual dose is 1 spray in each nostril once a day.
Do not use Nasacort for more than 3 months in children under 12 years old.
An adult should help a small child use this medicine.
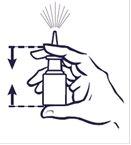
How to use the spray
Before you use your nasal spray, gently blow your nose to clear your nostrils.
|
|
|
|
|
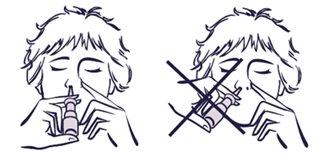
correct position incorrect position |
| |
| |
|
If you have not used the spray for more than 2 weeks:
- You need to prime the spray again, to refill the nozzle with spray.
- Point the nozzle away from you while you are doing this.
- For priming, you need to do one spray into the air, before you use it again.
- Always shake the bottle gently before use.
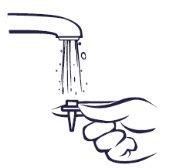
Cleaning the spray
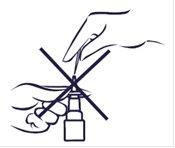
If the spray does not work, the nozzle may be blocked. Nevertry to unblock it or make the spray hole bigger with a pin or other sharp object. This could make the spray stop working.
You should clean the nasal spray at least once a week. You can clean it more often if it gets blocked.
Instructions for cleaning the spray:
| |
If you use more Nasacort than you should
It is important that you take your dose as instructed by your doctor or pharmacist. If you use more Nasacort than you should, talk to a doctor.
If you have used more Nasacort than you should, you may get stomach or intestine problems. If you have taken all the contents of the pack by mouth, talk to a doctor or go to the nearest hospital casualty department. Take the pack with you.
If you forget to use Nasacort
If you forget to use Nasacort, use it as soon as you remember. Do not use a double dose to make up for a forgotten dose.
If you stop using Nasacort
If you stop using this medicine, your symptoms may come back after a few days.
If you get any side effects after stopping this medicine, talk to your doctor. See section 4 “Possible side effects” for more information.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Nasacort and tell a doctor or go to a hospital straight away if:
- you have an allergic reaction to Nasacort. The symptoms can include: a rash (hives), itching, problems swallowing or breathing, swelling of the lips, face, throat or tongue.
Common side effects(may affect up to 1 in 10 people)
- Nasal discharge, headache, sore throat and/or cough
- Nosebleeds
- Inflammation/irritation of the airways (bronchitis)
- Heartburn or indigestion
- Flu-like symptoms (fever, muscle pain, weakness and/or fatigue)
- Dental problems
Other side effects(frequency not known: cannot be estimated from the available data)
- Irritation and dryness inside your nose
- Congestion or blockage of the nasal passages
- Sneezing
- Changes in the way things taste and smell
- Feeling sick (nausea)
- Sleep problems, feeling dizzy or tired
- Shortness of breath (dyspnoea)
- Low levels of cortisol in the blood (blood test results)
- Cloudy vision (cataracts), high pressure in the eye (glaucoma)
- Blurred vision.
- Stopping corticosteroids suddenly after a long time of use can lead to a steroid withdrawal syndrome. Symptoms of withdrawal such as joint pain, muscle pain, tremors, weight loss, anxiety, runny and bleeding nose can occur with intranasal triamcinolone. However, these symptoms are extremely rare with nasal steroid sprays, and are much less likely to occur with nasal steroid sprays than with steroid tablets or injections.
In some people, Nasacort can damage the inside of the nose in the middle (called the “nasal septum”). Talk to your doctor or pharmacist if you have any questions about this.
Other side effects in children
If your child has been using this medicine, it may affect their growth. This means your doctor will need to check their height from time to time. Your doctor may reduce the dose. Your doctor may also consider referring you to a specialist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nasacort
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and bottle after “EXP”. The expiry date refers to the last day of that month.
- Do not store above 25°C.
- After opening Nasacort for the first time, the 30 spray pack size should be used within 1 month and the 120 spray pack size within 2 months.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Nasacort Composition
The active ingredient is triamcinolone acetonide. One application contains 55 micrograms of triamcinolone acetonide.
The other components are:
- microcrystalline cellulose and sodium carmellose (dispersible cellulose)
- polysorbate 80
- purified water
- anhydrous glucose
- benzalkonium chloride (50% w/v solution),
- disodium edetate
- hydrochloric acid or sodium hydroxide (for pH adjustment)
Product Appearance and Container Content
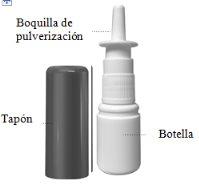
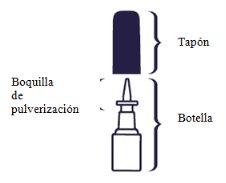
Nasacort is a suspension for nasal spray. It is presented in a white plastic bottle with a pump to deliver the spray through a nozzle. The bottle has a protective cap to keep the nozzle clean and to prevent accidental sprays.
This single bottle contains at least 120 sprays (16.5 g of suspension with 9.075 mg of triamcinolone acetonide) or at least 30 sprays (6.5 g of suspension with 3.575 mg of triamcinolone acetonide).
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Opella Healthcare Spain, S.L.
C/ Rosselló i Porcel, 21
08016 Barcelona
Spain
Sanofi Group
Manufacturer:
Bespak HC Limited London Road, Holmes Chapel
Cheshire, CW4 8BE
United Kingdom
or
Sanofi Winthrop Industrie
30-36, avenue Gustave Eiffel
37100 Tours
France
or
Sanofi-Aventis Deutschland GmbH
65926 Frankfurt am Main
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names: Nasacort
Austria: Nasacort Nasenspray
Belgium: Nasacort
Bulgaria: Nasacort
Czech Republic: Triamcinolone sanofi 55 micrograms/dose
Denmark: Nasacort
Estonia: Nasacort
Finland: Nasacort
Germany: Nasacort 55 micrograms/dose
Greece: Nasacort
Hungary: Nasacort
Ireland: Nasacort nasal spray
Italy: Nasacort
Latvia: Nasacort
Lithuania: Triamcinolone acetonide Sanofi 55 μg/dose nasal spray, suspension
Luxembourg: Nasacort
Netherlands: Nasacort
Poland: Nasacort
Portugal: Nasacort
Romania: Nasacort 55 micrograms/dose nasal spray, suspension
Slovakia: Nasacort 55 micrograms/dose
Slovenia: Nasacort
Spain: Nasacort 55 micrograms/dose, nasal spray suspension
Sweden: Nasacort
United Kingdom: Nasacort nasal spray
Date of the last revision of this leaflet: August 2022
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price15.55 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NASACORT 55 micrograms/dose, NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: NASAL PRODUCT, 27.5 MICROGRAMS/SPRAYActive substance: fluticasone furoateManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for NASACORT 55 micrograms/dose, NASAL SPRAY SUSPENSION
Discuss questions about NASACORT 55 micrograms/dose, NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions