

Feiba Nf

Ask a doctor about a prescription for Feiba Nf

How to use Feiba Nf
Package Leaflet: Information for the User
FEIBA NF, 500 units (50 units/ml), powder and solvent for solution for injection
Anti-inhibitor Coagulant Complex
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Package Leaflet
- 1. What FEIBA NF is and what it is used for
- 2. Important information before using FEIBA NF
- 3. How to use FEIBA NF
- 4. Possible side effects
- 5. How to store FEIBA NF
- 6. Contents of the pack and other information
1. What FEIBA NF is and what it is used for
FEIBA NF is a medicine obtained from human plasma, which enables blood clotting in cases of deficiency or absence of certain clotting factors.
FEIBA NF is used to treat bleeding and prevent bleeding in patients with hemophilia A complicated by the presence of factor VIII inhibitors and hemophilia B complicated by the presence of factor IX inhibitors.
Additionally, FEIBA NF may be used to treat and prevent bleeding in non-hemophilic patients with acquired inhibitors of factors VIII, IX, and XI.
FEIBA NF is also used in combination with factor VIII concentrate during long-term treatment aimed at complete and permanent elimination of factor VIII inhibitors, to enable regular treatment with factor VIII concentrate, as in patients without inhibitors.
In isolated cases, FEIBA NF has been used in patients with factor von Willebrand inhibitor.
2. Important information before using FEIBA NF
Inform your doctor if you have any allergies.
Inform your doctor if you are on a low-sodium diet.
When not to use FEIBA NF
In the following situations, FEIBA NF should not be used if alternative treatment is possible:
- if the patient is hypersensitive to the anti-inhibitor coagulant complex or any of the other components of this medicine (listed in section 6).
- if the patient has disseminated intravascular coagulation (DIC, consumption coagulopathy, a life-threatening condition associated with massive blood clotting and consumption of clotting factors).
- if the patient has acute thrombosis or embolism (including myocardial infarction).
See section "Warnings and precautions".
Warnings and precautions
Discuss the use of FEIBA NF with your doctor before starting treatment.
This medicine may cause allergic-type hypersensitivity reactions, including hives, angioedema, gastrointestinal symptoms, bronchospasm, and hypotension; these reactions can be severe and systemic (e.g., anaphylactic reaction with hives and angioedema, bronchospasm, and shock). Other infusion-related reactions, such as chills, fever, and hypertension, have also been reported.
If the first signs or symptoms of an infusion-related reaction/hypersensitivity reaction occur (see section 4), administration of this medicine should be discontinued and appropriate medical care should be initiated.
In patients with suspected hypersensitivity to the medicine or any of its components, the doctor will decide on re-administration of FEIBA NF only after careful consideration of the risk and expected benefits and (or) when no alternative preventive or alternative therapeutic measures can be expected.
During treatment with FEIBA NF, thromboembolic events, including disseminated intravascular coagulation (DIC), venous thrombosis, pulmonary embolism, myocardial infarction, and stroke, have occurred.
If the first signs or symptoms of thromboembolic events occur (see section 4), the infusion should be discontinued immediately and appropriate diagnostic and therapeutic measures should be initiated.
Some thromboembolic events have occurred with the use of high doses of FEIBA NF or in patients with other risk factors for thromboembolic events, including DIC, advanced atherosclerosis, crush injury, or sepsis. Concomitant use of recombinant factor VIIa may increase the risk of thromboembolic events. In patients with congenital or acquired hemophilia, the possibility of such risk factors should always be considered.
The medicine should be used with caution in patients at risk of DIC, arterial or venous thrombosis.
Cases of thrombotic microangiopathy have been reported in a clinical study of emicizumab, in which patients received FEIBA NF as part of the breakthrough bleeding treatment regimen.
In patients with hemophilia and inhibitors or acquired inhibitors of clotting factors, during treatment with FEIBA NF, there may be an increased tendency to bleeding and an increased risk of thrombosis.
In the following situations, the use of FEIBA NF is only justified when no response to treatment with the appropriate clotting factor concentrate is expected, e.g., in the case of high-titer inhibitors and life-threatening bleeding or risk of bleeding (e.g., post-traumatic or post-operative):
- Disseminated intravascular coagulation (DIC).
- Liver damage: due to delayed elimination of active clotting factors in patients with impaired liver function, there is an increased risk of DIC.
- Coronary heart disease, acute thrombosis, and (or) embolism. See section "When not to use FEIBA NF".
In the case of medicines produced from human blood or plasma, certain measures are taken to prevent infections in patients. These measures include careful selection of blood and plasma donors to ensure that those at risk of transmitting infections are excluded, as well as testing of individual blood samples and plasma pools for viruses/infections. Manufacturers of these products also include steps in the blood and plasma processing process that are designed to inactivate or remove viruses. Despite these measures, when administering medicines produced from human blood or plasma, it is not possible to completely exclude the possibility of transmitting an infection. This applies to both known and newly discovered viruses or other types of infections.
Measures are considered effective against enveloped viruses, such as HIV (which causes AIDS), hepatitis B virus, and hepatitis C virus, as well as non-enveloped hepatitis A virus. Measures may have limited value against non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be serious in pregnant women (fetal infection) and in individuals with impaired immune function or increased erythropoiesis (e.g., hemolytic anemia).
In patients receiving regular or repeated factor VIII from human plasma, consideration should be given to appropriate vaccinations (against viral hepatitis A and B).
After administration of high doses of FEIBA NF, a transient increase in passively transferred antibodies against the hepatitis B surface antigen may result in false-positive serological test results.
The medicine contains isoagglutinins, antibodies against red blood cells, which, when passively transferred, may affect the results of serological tests for the presence of antibodies against red blood cells, such as the antiglobulin test (Coombs test).
Each time a dose of FEIBA NF is administered to a patient, the name and batch number of the medicine should be clearly recorded to maintain information on the batches used.
FEIBA NF and other medicines
Inform your doctor about all medicines you are currently taking or have recently taken, including those available without a prescription.
No adequate and well-controlled studies have been conducted on the concomitant or sequential use of FEIBA NF and recombinant factor VIIa, fibrinolysis inhibitors, or emicizumab (see "Warnings and precautions").
The possibility of thromboembolic events should be considered when using systemic fibrinolysis inhibitors, such as tranexamic acid and aminocaproic acid, in combination with FEIBA NF. Therefore, fibrinolysis inhibitors should not be used for approximately 6 to 12 hours after administration of FEIBA NF.
Based on available in vitro data and clinical observations, in cases of concomitant use of recombinant factor VIIa, a potential drug interaction cannot be excluded, which may result in a thromboembolic event.
If treatment with FEIBA NF is considered after the patient has received emicizumab, the patient should be closely monitored by the treating physician.
As with all coagulation factor products, FEIBA NF should not be mixed with other medicines before administration; this may adversely affect the efficacy and safety of the medicine.
It is recommended to flush the venous access with isotonic sodium chloride solution before and after administration of FEIBA NF.
Pregnancy, breastfeeding, and fertility
The doctor will decide whether FEIBA NF can be used during pregnancy or breastfeeding. Due to the increased risk of thrombosis during pregnancy, FEIBA NF should only be used under close medical supervision and when clearly indicated.
Regarding the risk of parvovirus B19 infection, see "Warnings and precautions".
Driving and using machines
No effects on the ability to drive and use machines have been observed.
FEIBA NF contains sodium
The medicine contains 40 mg of sodium (the main component of common salt) per vial. This corresponds to 2% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use FEIBA NF
The lyophilized powder FEIBA NF is dissolved in the supplied solvent and administered intravenously.
This medicine should always be used exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist.
Treatment should be initiated and supervised by a doctor experienced in the treatment of coagulation disorders.
The doctor will determine the appropriate dose and frequency of administration individually for each patient, taking into account the severity of the coagulation disorder, the location and extent of bleeding, and the patient's overall condition and response to the medicine. Do not change the dose of the medicine yourself or stop treatment without consulting your doctor.
If you feel that the medicine is too strong or too weak, contact your doctor or pharmacist.
Before administration, the medicine should be warmed to room temperature or body temperature if necessary.
FEIBA NF should be prepared immediately before administration.
The prepared solution should be used immediately (the medicine does not contain preservatives).
Gently mix by rotating until the powder is dissolved. Ensure that FEIBA NF is completely dissolved; otherwise, fewer units of FEIBA will pass through the filter of the device.
Do not use a solution that is cloudy or contains sediment. Do not use a solution from previously opened vials.
Only use the supplied solvent (sterile water for injection) and the set for preparing the solution.
If a different set for preparing and administering the solution is used than the one supplied with FEIBA NF, ensure that a filter with a pore size of at least 149 µm is used.
Do not use if the system maintaining sterility or the packaging of the medicine is damaged or compromised.
Record the administration of the medicine on the attached adhesive label.
Any unused medicine or waste material should be disposed of in accordance with local regulations.
Administration using needles:
Preparing the injection solution
Follow aseptic procedures throughout the procedure.
- 1. Warm the closed vial containing the solvent (sterile water for injection) to room temperature if necessary, e.g., by using a water bath for a few minutes (max. 37°C).
- 2. Remove the protective caps from the vials of powder and solvent and disinfect the rubber stoppers of both vials.
- 3. By twisting and pulling, remove the sheath from one end of the supplied double-ended needle (Fig. A). Insert the exposed needle into the rubber stopper of the vial with the solvent (Fig. C).
- 4. Remove the sheath from the other end of the double-ended needle, taking care not to touch the exposed part.
- 5. Invert the vial with the solvent and insert the free end of the double-ended needle into the rubber stopper of the vial with the powder (Fig. D). The solvent will be drawn into the vial with the powder by vacuum.
- 6. Separate the two vials by withdrawing the needle from the vial with the powder (Fig. E). Gently swirl or rotate the vial with the powder to facilitate dissolution.
- 7. After the powder has dissolved, insert the venting needle (Fig. F) and the foam will disappear. Remove the venting needle.
Injection/infusion
Follow aseptic procedures throughout the procedure.
- 1. Remove the sheath from the supplied needle with a filter by twisting and pulling, and attach the needle to a sterile single-use syringe. Draw the solution into the syringe (Fig. G).
- 2. Detach the needle with the filter from the syringe and, after attaching the supplied infusion set with a butterfly needle (or a single-use injection needle), slowly inject the solution intravenously. A syringe pump can be used to control the infusion rate.
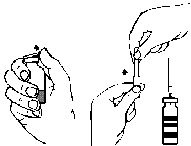
Fig. A Fig. B Fig. C Fig. D Fig. E Fig. F Fig. G
Do not exceed a rate of 2 FEIBA units per kg body weight per minute. Administration using BAXJECT II Hi-Flow:
Preparing the injection solution
Follow aseptic procedures throughout the procedure.
- 1. Bring the vial with the solvent (sterile water for injection) to room temperature if necessary, e.g., by using a water bath for a few minutes (max. 37°C).
- 2. Remove the protective caps from the vials of powder and solvent and disinfect the rubber stoppers of both vials. Place the vials on a flat surface.
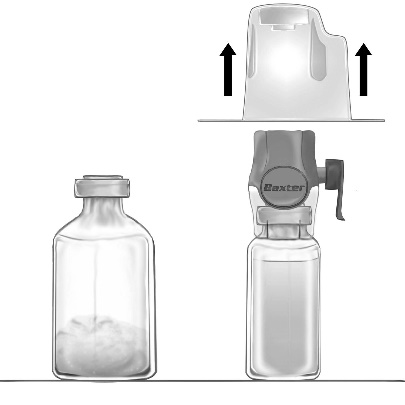
- 3. Open the packaging of the BAXJECT II Hi-Flow device by tearing off the paper cover, without touching the inside (Fig. a). Do not remove the device from the packaging.
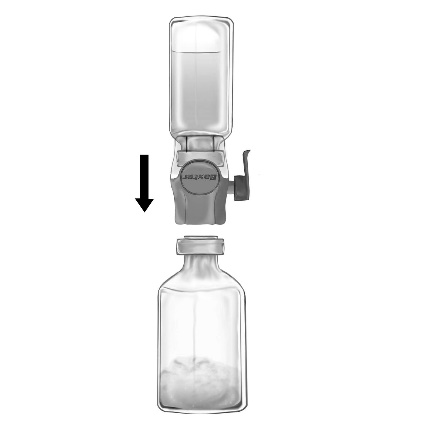
- 4. Invert the packaging and puncture the transparent plastic spike through the stopper of the solvent vial (Fig. b). Holding the packaging by the edges, remove it from the BAXJECT II Hi-Flow device (Fig. c). Do not remove the blue cap from the BAXJECT II Hi-Flow device.
BAXJECT II Hi-Flow (Fig. c)
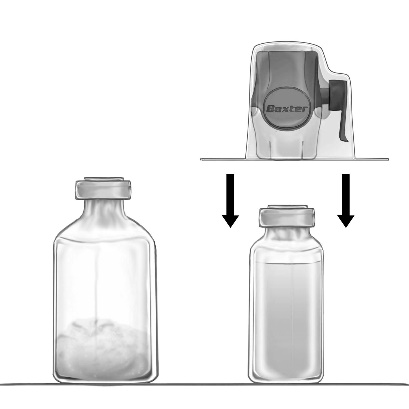
- 5. With the BAXJECT II Hi-Flow device connected to the solvent vial, invert the system so that the solvent vial is above the device. Puncture the purple plastic spike through the stopper of the vial with FEIBA NF powder. Under vacuum, the solvent will be drawn into the vial with FEIBA NF powder (Fig. d).
- 6. Mix gently, by rotating, but without shaking, until the product is completely dissolved. Ensure that FEIBA NF is completely dissolved – otherwise, the active substance will not pass through the filter of the device.
Fig. a
Fig. b
Fig. c

Injection/infusion
Follow aseptic procedures throughout the procedure.
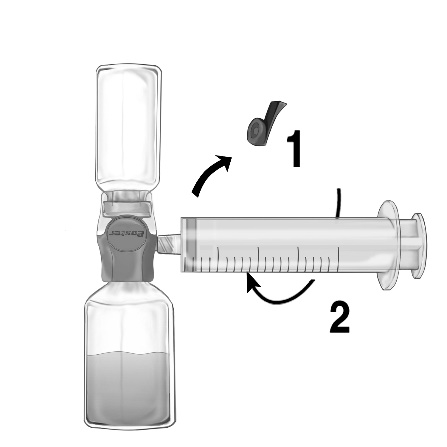
- 1. Remove the blue cap from the BAXJECT II Hi-Flow device. Securely connect the syringe to the BAXJECT II Hi-Flow device (DO NOT DRAW AIR INTO THE SYRINGE). To ensure a secure connection between the syringe and the BAXJECT II Hi-Flow device, it is particularly recommended to use a syringe with a luer-type tip (by screwing the syringe in a clockwise direction until it stops) (Fig. e).
- 2. Invert the system so that the solution is at the top. Draw the solution into the syringe, SLOWLY pulling the plunger back and ensuring that the connection between the syringe and the BAXJECT II Hi-Flow device is secure and the syringe is attached throughout the solution draw (Fig. f).
- 3. Detach the syringe.
- 4. If a foamy solution appears in the syringe, wait until the foam disappears. Slowly administer the solution intravenously using the infusion set (or a single-use injection needle). A syringe pump can be used to control the infusion rate.
Fig. d
Fig. e
Fig. f
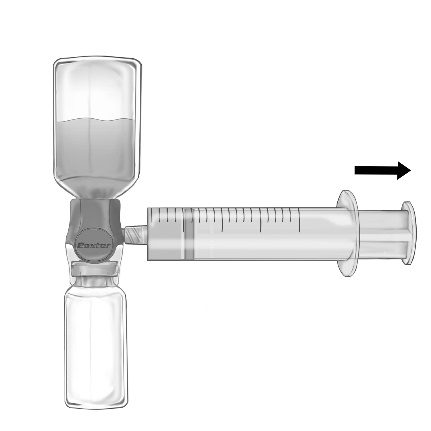
Do not exceed a rate of 2 FEIBA units per kg body weight per minute.
Overdose of FEIBA NF
Inform your doctor immediately. Overdose of FEIBA NF may increase the risk of side effects, such as thromboembolic events (formation of blood clots that can move through the blood vessels), consumption coagulopathy (a disorder of blood clotting), or myocardial infarction.
4. Possible side effects
Like all medicines, FEIBA NF can cause side effects, although not everybody gets them.
Inform your doctor immediatelyif you experience one or more of the following symptoms:
- Hypersensitivity reactions: rash, itching rash (hives), itching, swelling of the lips and tongue, wheezing, feeling of pressure in the chest, gastrointestinal symptoms, dizziness, sudden drop in blood pressure, chills, fever, hypertension
- Disseminated intravascular coagulation (DIC): spontaneous bruising, petechiae, severe, simultaneous bleeding (e.g., from wounds, injection sites, mucous membranes, genital tract), organ failure due to hypoperfusion (e.g., kidney failure - anuria or oliguria; lung failure - dyspnea, cough, hemoptysis; brain failure - disorientation and concentration problems, seizures, changes in consciousness, coma)
- Venous thrombosis: pain and swelling of the limb, usually unilateral, increased warmth of the limb, fever or subfebrile state
- Pulmonary embolism: significant changes in blood pressure or heart rate, difficulty breathing, cough or chest pain
- Myocardial infarction: chest pain that may radiate to the left arm or jaw, to the stomach or back; shortness of breath, palpitations, dizziness, fainting, weakness, anxiety, fear
- Stroke: sudden, severe headache, vision disturbances, unilateral drooping of the corner of the mouth, difficulty swallowing and speaking, coordination and balance disturbances, drowsiness, disorientation, loss of consciousness
Common side effects (may affect up to 1 in 10 people):
- Hypersensitivity
- Headache, dizziness
- Hypotension
- Rash
- Positive test result for antibodies against the hepatitis B surface antigen, elevated D-dimer fibrin levels
Side effects with unknown frequency (cannot be estimated from the available data):
- Disseminated intravascular coagulation (DIC), increase in inhibitor titer
- Anaphylactic reaction (a rapidly developing, life-threatening allergic reaction), itching rash (hives) all over the body
- Numbness (paresthesia), abnormal or decreased sensation, stroke (thrombotic or embolic stroke), drowsiness, taste disturbances
- Myocardial infarction (heart attack), palpitations (tachycardia)
- Thrombosis (venous or arterial thrombosis), which can move through the blood vessels (thromboembolic events), increased blood pressure (hypertension), sudden flushing
- Pulmonary embolism (pulmonary thromboembolism), bronchospasm, wheezing, cough, shortness of breath (dyspnea)
- Vomiting, diarrhea, abdominal discomfort, nausea
- Numbness of the face, facial swelling, tongue and lip swelling (angioedema), itching rash (hives) all over the body, itching (pruritus)
- Pain at the injection site, general malaise, feeling of heat, chills, fever, chest pain, chest discomfort
- Drop in blood pressure
Symptoms of hypersensitivity reactions after administration of medicines derived from human plasma also include coma and restlessness.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store FEIBA NF
Keep the medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Store the medicine in the outer packaging to protect it from light.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What FEIBA NF contains
Powder
- The active substance is an anti-inhibitor coagulant complex. After reconstitution in 10 ml of the supplied solvent (water for injection), 1 ml contains approximately 50 units of anti-inhibitor coagulant complex. 1 vial contains 500 units of factor VIII with bypassing activity in 200-600 mg of human plasma protein.
- FEIBA NF also contains factors II, IX, and X, mainly in the form of non-activated factors, as well as activated factor VII. Antigenic factor VIII (F VIII C:Ag) is present in a concentration of up to 0.1 unit per 1 unit of FEIBA. The factors of the kallikrein-kinin system are present only in trace amounts or not at all.
- Other ingredients of the medicine are sodium chloride and sodium citrate.
Solvent
- water for injection
What FEIBA NF looks like and contents of the pack
The medicine is a lyophilized powder or a fine white or pale green solid.
The powder and solvent are supplied in glass vials sealed with rubber stoppers.
The pH of the solution after reconstitution is between 6.8 and 7.6.
Package size: 1 set
Contents of the pack (with needles):
1 vial with 500 units of FEIBA NF, sealed with a rubber stopper
1 vial with 10 ml of water for injection, sealed with a rubber stopper
1 double-ended needle
1 venting needle
1 single-use syringe
1 injection needle
1 needle with a filter
1 butterfly needle (infusion set with butterfly needle)
Contents of the pack (with BAXJECT II Hi-Flow):
1 vial with 500 units of FEIBA NF, sealed with a rubber stopper
1 vial with 10 ml of water for injection, sealed with a rubber stopper
1 BAXJECT II Hi-Flow - a needleless transfer device for transferring and mixing medicines contained in two vials
1 single-use syringe
1 injection needle
1 butterfly needle (infusion set with butterfly needle)
Marketing authorization holder and manufacturer
Marketing authorization holder:
Takeda Pharma Sp. z o.o.
ul. Prosta 68
00-838 Warsaw
Phone: +48 22 306 24 47
[email protected]
Manufacturer:
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Vienna, Austria
Date of last revision of the package leaflet:
-----------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Treatment should be initiated and supervised by a doctor experienced in the treatment of hemophilia.
Dosage
Dosage and duration of treatment depend on the severity of the coagulation disorder, the location and extent of bleeding, and the patient's clinical condition.
Dose, as well as frequency of administration, should always be aimed at clinical efficacy in the given case.
Generally, a dose of 50-100 units of FEIBA per kg body weight is recommended; however, the single dose should not exceed 100 units/kg body weight, and the maximum daily dose should not exceed 200 units/kg body weight, unless the severity of bleeding requires and justifies the use of higher doses.
Due to specific factors for the given patient, the response to the anti-inhibitor coagulant complex may vary, and in a given case of bleeding in a patient in whom the response to one of the factors is insufficient, the use of another factor should be considered.
Children and adolescents
Experience with the use of FEIBA NF in children under 6 years of age is limited; the dosing schedule, the same as for adults, should be adjusted according to the child's clinical condition.
- 1)
Spontaneous bleeding
Bleeding into joints, muscles, and soft tissues
In cases of minor or moderate bleeding, a dose of 50-75 units/kg body weight is recommended at 12-hour intervals. Treatment should be continued until clear signs of clinical improvement are observed, such as relief of pain, reduction of swelling, or mobilization of the joint.
In cases of major bleeding into muscles and soft tissues, such as bleeding into the retroperitoneal space, doses of 100 units/kg body weight at 12-hour intervals are recommended.
Bleeding from mucous membranes
A dose of 50 units/kg body weight every 6 hours is recommended, with close monitoring of the patient (observation of the bleeding surface, repeated hematocrit measurements in the patient). If bleeding does not stop, the dose can be increased to 100 units/kg body weight. Do not exceed the maximum daily dose of 200 units/kg body weight.
Other severe bleeding
In severe bleeding, such as bleeding into the central nervous system, a dose of 100 units/kg body weight at 12-hour intervals is recommended. In individual cases, FEIBA NF can be administered at 6-hour intervals until clear clinical improvement is observed. Do not exceed the maximum daily dose of 200 units/kg body weight!
- 2)
Surgical procedures
Administer 50-100 units/kg body weight at 6-hour intervals, taking care not to exceed the maximum daily dose.
- 3)
Prophylaxis
There are limited clinical data on the use of FEIBA NF for prophylaxis of bleeding in patients with hemophilia.
- Prophylactic treatment of bleeding in patients with high-titer inhibitors and frequent bleeding, in whom induction of immune tolerance (ITI) has failed or is not considered: A dose of 70-100 units/kg body weight every other day is recommended. If bleeding in the patient does not stop, the dose can be increased to 100 units/kg body weight administered daily, or gradually decreased.
- Prophylactic treatment of bleeding in patients with high-titer inhibitors during induction of immune tolerance (ITI): FEIBA NF can be administered concomitantly with factor VIII concentrates at doses of 50-100 units/kg body weight twice daily, until the factor VIII inhibitor titer decreases to <2 bu.*< li>
*1 Bethesda unit is defined as the amount of antibody that reduces the activity of factor VIII by 50% in fresh normal human plasma after 2 hours of incubation at 37°C.
Method of administration
See also section "FEIBA NF and other medicines" and section 3 of the package leaflet.
FEIBA NF should be administered slowly intravenously (no faster than 2 units/kg body weight per minute).
FEIBA NF should be prepared immediately before administration.
The solution should be used immediately (the medicine does not contain preservatives).
Do not use a solution that is cloudy or contains sediment. Do not use if the needleless transfer device or double-ended needle, the system maintaining their sterility, or their packaging is damaged or compromised.
Unused solution should be disposed of in accordance with the applicable procedure.
Monitoring of treatment
Due to the complex mechanism of action, there is no direct method for monitoring the active substances.
Results of laboratory tests in vitro to assess the efficacy of treatment, such as aPTT, blood clotting time, and thromboelastogram (TEG), may not reflect clinical improvement. Therefore, attempts to restore normal values of these parameters by increasing the dose of FEIBA NF may be misleading and should be avoided due to the risk of DIC caused by overdose.
In cases of insufficient response to treatment with FEIBA NF, it is recommended to determine the platelet count, as a sufficient number of functionally intact platelets is necessary for the efficacy of FEIBA NF.
Do not exceed single doses of 100 units/kg body weight and a daily dose of 200 units/kg body weight. Patients receiving more than 100 units/kg body weight should be monitored for the occurrence of DIC and (or) acute coronary syndrome. High doses of FEIBA NF should only be administered for the period necessary to stop bleeding.
In cases of significant changes in blood pressure, heart rate, respiratory disturbances, chest pain, and cough, administration of the medicine should be discontinued immediately and appropriate diagnostic and therapeutic measures should be initiated.
Laboratory results indicating DIC include decreased fibrinogen levels, decreased platelet count, and the presence of fibrin/fibrinogen degradation products (FDP).
Administration of FEIBA NF to patients with inhibitors may result in an initial anamnestic increase in inhibitor levels. Under the influence of further administration of FEIBA NF, the inhibitor level may decrease over time. Clinical and literature data indicate that the efficacy of FEIBA NF is not reduced.
During administration of FEIBA NF, in patients with hemophilia complicated by the presence of inhibitors or in patients with acquired inhibitors of clotting factors, there may be a simultaneous tendency to bleeding and an increased risk of thrombosis.
See also section "Warnings and precautions".
Detailed information on this medicine is available in the Summary of Product Characteristics available on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterTakeda Manufacturing Austria AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Feiba NfDosage form: Powder, 500 IU = 500 IU FEIBAActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription requiredDosage form: Powder, 1000 IU (1000 IU FEIBA), 50 IU/mlActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription requiredDosage form: Powder, 2500 IU (2500 IU FEIBA), 50 IU/mlActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription required
Alternatives to Feiba Nf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Feiba Nf in Spain
Online doctors for Feiba Nf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Feiba Nf – subject to medical assessment and local rules.














