

Feiba Nf

Ask a doctor about a prescription for Feiba Nf

How to use Feiba Nf
Package Leaflet: Information for the User
FEIBA NF, 2500 units (50 units/ml), powder and solvent for solution for injection
Anti-inhibitor Coagulant Complex
This is a complex of clotting factors that counteracts inhibitors of factor VIII.
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this package leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Package Leaflet
- 1. What is FEIBA NF and what is it used for
- 2. Important information before using FEIBA NF
- 3. How to use FEIBA NF
- 4. Possible side effects
- 5. How to store FEIBA NF
- 6. Contents of the pack and other information
1. What is FEIBA NF and what is it used for
FEIBA NF is a medicine obtained from human plasma, which allows blood to clot in cases of deficiency or lack of certain clotting factors.
FEIBA NF is used to treat bleeding and prevent bleeding in patients with hemophilia A complicated by the presence of factor VIII inhibitors and hemophilia B complicated by factor IX inhibitors.
Additionally, FEIBA NF may be used to treat and prevent bleeding in non-hemophilic patients with acquired inhibitors of factors VIII, IX, and XI.
FEIBA NF is also used in combination with factor VIII concentrate during long-term treatment aimed at complete and permanent elimination of factor VIII inhibitors, to enable regular treatment with factor VIII concentrate, as in patients without inhibitors.
In isolated cases, FEIBA NF has been used in patients with von Willebrand factor inhibitors.
2. Important information before using FEIBA NF
Inform your doctor if you have any allergies.
Inform your doctor if you are on a low-sodium diet.
When not to use FEIBA NF
In the following situations, FEIBA NF should not be used if alternative treatment is possible:
- If the patient is hypersensitive to the anti-inhibitor coagulant complex or any of the other components of this medicine (listed in section 6).
- If the patient has disseminated intravascular coagulation (DIC, consumption coagulopathy, a life-threatening condition associated with massive blood clotting and consumption of clotting factors).
- If the patient has acute thrombosis or embolism (including myocardial infarction).
See "Warnings and precautions" section.
Warnings and precautions
Discuss the use of FEIBA NF with your doctor before starting treatment.
This medicine may cause allergic reactions, including hives, angioedema, gastrointestinal symptoms, bronchospasm, and hypotension; these reactions can be severe and systemic (e.g., anaphylactic reaction with hives and angioedema, bronchospasm, and shock). Other infusion-related reactions, such as chills, fever, and hypertension, have also been reported.
If signs or symptoms of infusion-related reactions or allergic reactions occur (see section 4), discontinue the administration of this medicine and initiate appropriate medical care.
In patients suspected of being hypersensitive to this medicine or any of its components, the doctor will decide on re-administration of FEIBA NF only after careful consideration of the risk and expected benefits and (or) when no alternative preventive or alternative therapeutic measures can be expected.
During treatment with FEIBA NF, thrombotic events, including disseminated intravascular coagulation (DIC), venous thrombosis, pulmonary embolism, myocardial infarction, and stroke, have occurred.
If signs or symptoms of thrombotic events occur (see section 4), discontinue the infusion immediately and initiate appropriate diagnostic and therapeutic measures.
Some thrombotic events have occurred with high doses of FEIBA NF or in patients with other risk factors for thrombotic events, including DIC, advanced atherosclerosis, crush injury, or sepsis. Concomitant use of recombinant factor VIIa may increase the risk of thrombotic events. In patients with congenital or acquired hemophilia, these risk factors should always be considered.
The medicine should be used with caution in patients at risk of DIC, arterial or venous thrombosis.
Cases of microangiopathic hemolytic anemia have been reported in a clinical study of emicizumab, in which patients received FEIBA NF as part of the breakthrough bleeding treatment regimen.
In patients with hemophilia and inhibitors or acquired inhibitors of clotting factors, treatment with FEIBA NF may be associated with an increased risk of bleeding and thrombosis.
- Disseminated intravascular coagulation (DIC).
- Liver damage: due to delayed elimination of active clotting factors in patients with impaired liver function, there is an increased risk of DIC.
- Coronary heart disease, acute thrombosis, and (or) embolism. See "When not to use FEIBA NF" section.
For medicines produced from human blood or plasma, certain measures are taken to prevent infections in patients. These measures include careful selection of blood and plasma donors to ensure that those at risk of transmitting infections are excluded, and testing of individual donations and plasma pools for viruses/infections. Manufacturers of these products also include steps in the processing of blood and plasma that are designed to inactivate or remove viruses. Despite these measures, the risk of transmitting infections cannot be completely excluded when administering medicines produced from human blood or plasma. This also applies to unknown or newly discovered viruses or other types of infections.
Measures taken are considered effective against enveloped viruses, such as HIV (which causes AIDS), hepatitis B virus, and hepatitis C virus, as well as non-enveloped hepatitis A virus. Measures taken may have limited value against non-enveloped viruses, such as parvovirus B19. Parvovirus B19 infection can be serious in pregnant women (fetal infection) and for individuals with impaired immune function or increased erythropoiesis (e.g., hemolytic anemia).
For patients receiving regular or repeated factor VIII from human plasma, consideration should be given to vaccination against hepatitis A and B.
After administration of high doses of FEIBA NF, a transient increase in passively transferred antibodies against the surface antigen of hepatitis B virus may result in false-positive serological test results.
The medicine contains isoagglutinins, antibodies against red blood cells, which, when passively transferred, may affect the results of serological tests for the presence of antibodies against red blood cells, such as the antiglobulin test (Coombs test).
Each time a dose of FEIBA NF is administered to a patient, the name and batch number of the medicine should be clearly recorded to maintain information on the batches used.
FEIBA NF and other medicines
Inform your doctor about all medicines you are currently taking or have recently taken, including those available without a prescription.
No adequate and well-controlled studies have been conducted on concomitant or sequential use of FEIBA NF and recombinant factor VIIa, antifibrinolytic agents, or emicizumab (see "Warnings and precautions").
The possibility of thrombotic events should be considered when using systemic antifibrinolytic agents, such as tranexamic acid and aminocaproic acid, in combination with FEIBA NF. Therefore, antifibrinolytic agents should not be used for approximately 6 to 12 hours after administration of FEIBA NF.
Based on available in vitro data and clinical observations, with concomitant use of recombinant factor VIIa, a potential drug interaction cannot be excluded, which may result in a thrombotic event.
If treatment with FEIBA NF is considered after a patient has received emicizumab, the patient should be closely monitored by the treating physician.
As with all clotting factor products, FEIBA NF should not be mixed with other medicines before administration; this may adversely affect the efficacy and safety of the medicine.
It is recommended to flush the intravenous access line with isotonic sodium chloride solution before and after administration of FEIBA NF.
Pregnancy, breastfeeding, and fertility
Your doctor will decide whether FEIBA NF can be used during pregnancy or breastfeeding. Due to the increased risk of thrombosis during pregnancy, FEIBA NF should only be used under close medical supervision and when clearly indicated.
Regarding the risk of parvovirus B19 infection, see "Warnings and precautions" section.
Driving and using machines
No effects on the ability to drive and use machines have been observed.
FEIBA NF contains sodium
The medicine contains 200 mg of sodium (the main component of common salt) per vial, which is approximately 10% of the maximum recommended daily intake of sodium in the diet for adults.
3. How to use FEIBA NF
FEIBA NF powder is dissolved in the provided solvent and administered intravenously.
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
Treatment should be initiated and monitored by a doctor experienced in the treatment of bleeding disorders.
Your doctor will determine the appropriate dose and frequency of administration individually for each patient, taking into account the severity of the bleeding disorder, the location and extent of the bleeding, and the patient's overall condition and response to the medicine. Do not change the dose of the medicine without consulting your doctor, and do not stop treatment without consulting your doctor.
If you feel that the medicine is too strong or too weak, contact your doctor or pharmacist.
Before administration, the medicine should be warmed to room temperature or body temperature if necessary.
FEIBA NF should be prepared immediately before use.
The prepared solution should be used immediately (the medicine does not contain preservatives). Gently mix by rotating until the powder is completely dissolved. Ensure that FEIBA NF is completely dissolved; otherwise, fewer units of FEIBA will pass through the filter of the administration device.
Do not use a solution that is cloudy or contains sediment. Do not use a solution from previously opened vials.
Use only the provided solvent (sterile water for injection) and the administration device.
If a different administration device is used than the one provided with FEIBA NF, ensure that a filter with a pore size of at least 149 µm is used.
Do not use if the administration device, its sterile system, or packaging is damaged or broken.
Record the administration of the medicine on the attached label.
Any unused solution or waste material should be disposed of in accordance with local regulations.
Preparing the solution for injection
Follow aseptic procedures throughout the procedure.
- 1. Bring the vial of solvent (sterile water for injection) to room temperature if necessary, e.g., by using a water bath for a few minutes (maximum 37°C).
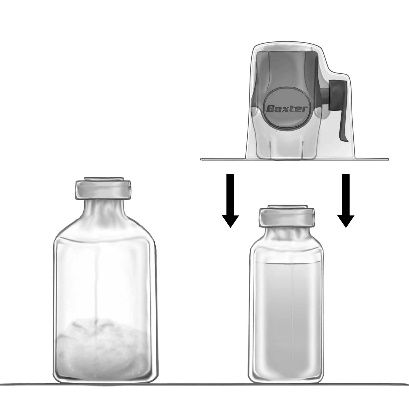
- 2. Remove the protective caps from the vials of powder and solvent and disinfect the rubber stoppers of both vials. Place the vials on a flat surface.
- 3. Open the packaging of the BAXJECT II Hi-Flow device by tearing off the paper cover, without touching the inside (Fig. a). Do not remove the device from its packaging.
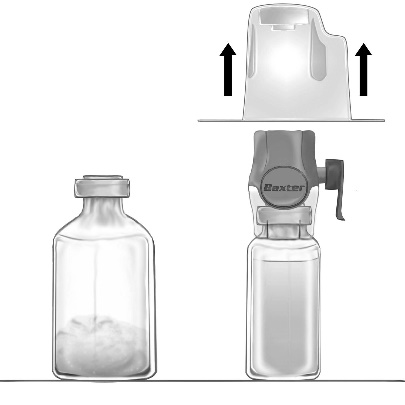
- 4. Turn the packaging upside down and puncture the transparent plastic spike through the stopper of the solvent vial (Fig. b). Holding the packaging by the edges, remove it from the BAXJECT II Hi-Flow device (Fig. c). Do not remove the blue cap from the BAXJECT II Hi-Flow device.
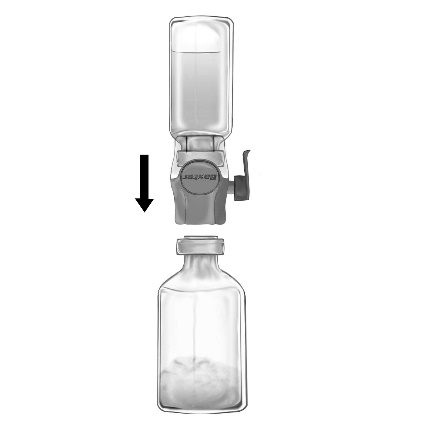
- 5. Turn the BAXJECT II Hi-Flow device connected to the solvent vial upside down so that the solvent vial is above the device. Puncture the purple plastic spike through the stopper of the FEIBA NF vial. Under vacuum, the solvent will be drawn into the FEIBA NF vial (Fig. d).
- 6. Mix gently by rotating, but without shaking, until the powder is completely dissolved. Ensure that FEIBA NF is completely dissolved; otherwise, the active substance will not pass through the filter of the administration device.
Fig. a
Fig. b
Fig. c

Injection/infusion
Follow aseptic procedures throughout the procedure.
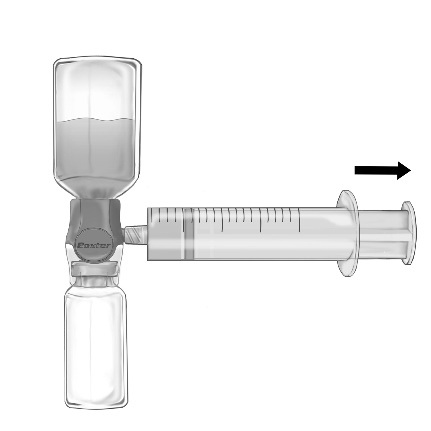
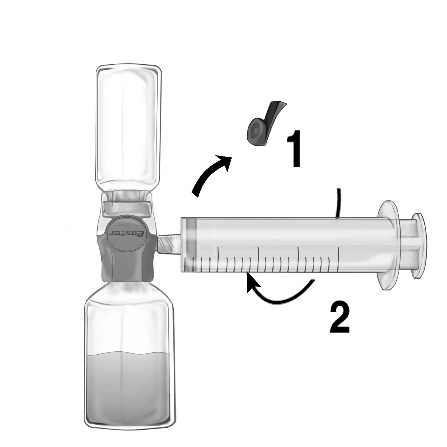
- 1. Remove the blue cap from the BAXJECT II Hi-Flow device. Securely connect the syringe to the BAXJECT II Hi-Flow device (DO NOT DRAW AIR INTO THE SYRINGE). To ensure a secure connection between the syringe and the BAXJECT II Hi-Flow device, it is recommended to use a syringe with a luer-type tip (by screwing the syringe clockwise until it stops) (Fig. e).
- 2. Turn the assembly upside down so that the solution is at the top. Draw the solution into the syringe by slowly pulling the plunger back and ensuring that the connection between the syringe and the BAXJECT II Hi-Flow device is secure and the syringe is held in place throughout the solution transfer (Fig. f).
- 3. Disconnect the syringe.
- 4. If a foamy solution appears in the syringe, wait until the foam disappears. Administer the solution slowly intravenously using an infusion set (or a single-use injection needle). A syringe pump can be used to control the infusion rate.
Fig. d
Fig. e
Fig. f
Do not exceed the infusion rate of 2 units of FEIBA per kg body weight per minute.
Overdose of FEIBA NF
Inform your doctor immediately. Overdose of FEIBA NF may increase the risk of side effects, such as thrombotic events (formation of blood clots that can travel through the blood vessels), consumption coagulopathy (a bleeding disorder), or myocardial infarction.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Inform your doctor immediatelyif you experience one or more of the following symptoms:
- Allergic reactions: rash, itching rash (hives), itching, swelling of the lips and tongue, wheezing, feeling of pressure in the chest, gastrointestinal symptoms, dizziness, sudden drop in blood pressure, chills, fever, hypertension, coma
- Disseminated intravascular coagulation (DIC): spontaneous bruising, petechiae, severe and simultaneous bleeding (e.g., from wounds, injection sites, mucous membranes, genital tract), organ failure due to inadequate blood supply (e.g., kidney failure - anuria or oliguria; lung failure - dyspnea, cough, hemoptysis; brain failure - disorientation and concentration problems, seizures, altered consciousness, coma)
- Venous thrombosis: pain and swelling of the limb, usually unilateral, increased warmth of the limb, low-grade fever or fever
- Pulmonary embolism: significant changes in blood pressure or heart rate, difficulty breathing, coughing, or chest pain
- Myocardial infarction: chest pain that may radiate to the left arm or shoulder, jaw, epigastric region, or back; shortness of breath, palpitations, dizziness, fainting, weakness, anxiety, fear
- Stroke: sudden severe headache, vision disturbances, unilateral facial weakness, difficulty swallowing and speaking, coordination and balance disturbances, drowsiness, disorientation, loss of consciousness
Frequent side effects (may affect up to 1 in 10 people):
- Hypersensitivity
- Headache, dizziness
- Hypotension
- Rash
- Positive test results for antibodies against the surface antigen of hepatitis B virus, elevated D-dimer levels
Side effects with unknown frequency (cannot be estimated from available data):
- Disseminated intravascular coagulation (DIC), increase in inhibitor titer
- Anaphylactic reaction (a rapidly developing, life-threatening allergic reaction), itching rash (hives) all over the body
- Numbness of the limbs (hypoesthesia), abnormal or decreased sensation (paresthesia), stroke (thrombotic or embolic stroke), drowsiness, taste disturbances
- Myocardial infarction (heart attack), palpitations (tachycardia)
- Formation of blood clots (venous or arterial thrombosis) that can travel through the blood vessels (thromboembolic events), increased blood pressure (hypertension), sudden flushing
- Pulmonary embolism (pulmonary thromboembolism), narrowing of the airways (bronchospasm), wheezing, coughing, shortness of breath (dyspnea)
- Vomiting, diarrhea, abdominal discomfort, nausea
- Numbness of the face, facial swelling, tongue and lip swelling (angioedema), itching rash (hives) all over the body, itching (pruritus)
- Pain at the injection site, general malaise, feeling of heat, chills, fever, chest pain, chest discomfort
- Hy potension
Symptoms of allergic reactions to medicines derived from human plasma also include coma and restlessness.
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store FEIBA NF
Store the medicine out of the sight and reach of children.
Do not store above 25°C. Do not freeze.
Store the medicine in the outer packaging to protect it from light.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What FEIBA NF contains
Powder
- The active substance is an anti-inhibitor coagulant complex. After reconstitution with 50 ml of the provided solvent (water for injection), 1 ml contains approximately 50 units of anti-inhibitor coagulant complex. One vial contains 2500 units of anti-inhibitor coagulant complex with a bypassing activity of 1000-3000 mg of human plasma protein.
- FEIBA NF also contains factors II, IX, and X, mainly in their non-activated form, as well as activated factor VII. Antigenic factor VIII (F VIII C:Ag) is present at a concentration of up to 0.1 unit per 1 unit of FEIBA. The kallikrein-kinin system factors are present in trace amounts or not at all.
- Other ingredients of the medicine are sodium chloride and sodium citrate.
Solvent
- Water for injection
What FEIBA NF looks like and contents of the pack
The medicine is a lyophilized powder or a fine white or pale green solid.
The powder and solvent are provided in glass vials sealed with rubber stoppers.
The pH of the solution after reconstitution is between 6.8 and 7.6.
Package size: 1 set
Contents of the pack:
1 vial with 2500 units of FEIBA NF, sealed with a rubber stopper
1 vial with 50 ml of water for injection, sealed with a rubber stopper
1 BAXJECT II Hi-Flow - a needleless transfer device for transferring and mixing the medicines in the two vials
1 syringe for single use
1 injection needle
1 butterfly needle (infusion set with butterfly needle)
Marketing authorization holder and manufacturer
Marketing authorization holder:
Takeda Pharma Sp. z o.o.
ul. Prosta 68
00-838 Warsaw
Phone: +48 22 306 24 47
[email protected]
Manufacturer:
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Vienna, Austria
Date of last revision of the package leaflet:
-----------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Treatment should be initiated and monitored by a doctor experienced in the treatment of hemophilia.
Dosage
Dosage and duration of treatment depend on the severity of the bleeding disorder, the location and extent of the bleeding, and the patient's clinical condition.
Dose and frequency of administration should always be aimed at achieving clinical efficacy in the individual case.
Generally, a dose of 50-100 units of FEIBA per kg body weight is recommended; however, the dose should not exceed 100 units/kg body weight per administration, or a maximum daily dose of 200 units/kg body weight, unless the severity of the bleeding requires and justifies the use of higher doses.
Due to specific factors for the individual patient, the response to the anti-inhibitor coagulant complex may vary, and in a given case of bleeding in a patient in whom the response to one of the factors is inadequate, the use of another factor should be considered.
Children and adolescents
Experience with the use of FEIBA NF in children under 6 years of age is limited; the dosing schedule, the same as for adults, should be adjusted according to the child's clinical condition.
- 1)
Spontaneous bleeding
Bleeding into joints, muscles, and soft tissues
In cases of minor or moderate bleeding, a dose of 50-75 units/kg body weight is recommended at 12-hour intervals. Treatment should be continued until clear signs of clinical improvement are observed, such as relief of pain, reduction of swelling, or mobilization of the joint.
In cases of severe bleeding into muscles and soft tissues, such as bleeding into the retroperitoneal space, a dose of 100 units/kg body weight is recommended at 12-hour intervals.
Bleeding from mucous membranes
A dose of 50 units/kg body weight is recommended every 6 hours, with close monitoring of the patient (monitoring of the bleeding surface, repeated hematocrit measurements in the patient). If bleeding does not stop, the dose can be increased to 100 units/kg body weight. Do not exceed the maximum daily dose of 200 units/kg body weight.
Other severe bleeding
In severe bleeding, such as bleeding into the central nervous system, a dose of 100 units/kg body weight is recommended at 12-hour intervals. In individual cases, FEIBA NF may be administered at 6-hour intervals until clear signs of clinical improvement are observed. Do not exceed the maximum daily dose of 200 units/kg body weight.
- 2)
Surgical procedures
Administer 50-100 units/kg body weight at 6-hour intervals, taking care not to exceed the maximum daily dose.
- 3)
Prophylaxis
There are limited clinical data on the use of FEIBA NF for prophylaxis of bleeding in patients with hemophilia.
- Prophylactic treatment of bleeding in patients with high inhibitor titers and frequent bleeding, in whom induction of immune tolerance (ITI) has failed or is not considered: A dose of 70-100 units/kg body weight every other day is recommended. If bleeding in the patient does not stop, the dose can be increased to 100 units/kg body weight administered daily, or gradually decreased.
- Prophylactic treatment of bleeding in patients with high inhibitor titers during induction of immune tolerance (ITI): FEIBA NF can be administered concomitantly with factor VIII concentrates at doses of 50-100 units/kg body weight twice daily, until the factor VIII inhibitor titer decreases to <2 bethesda units.< li>
*1 Bethesda unit is defined as the amount of antibody that reduces the activity of factor VIII by 50% in fresh normal human plasma after 2 hours of incubation at 37°C.
Method of administration
See also "FEIBA NF and other medicines" and section 3 of the package leaflet.
FEIBA NF should be administered slowly intravenously (no faster than 2 units/kg body weight per minute).
FEIBA NF should be prepared immediately before use.
The solution should be used immediately (it does not contain preservatives).
Do not use a solution that is cloudy or contains sediment.
Do not use if the needleless transfer device, its sterile system, or packaging is damaged or broken.
Unused solution should be disposed of in accordance with local regulations.
Monitoring of treatment
Due to the complex mechanism of action, there is no direct method for monitoring the active substances.
Laboratory test results in vitro to assess the efficacy of treatment, such as aPTT, blood clotting time, and thromboelastogram (TEG), may not reflect clinical improvement. Therefore, attempts to normalize these parameters by increasing the dose of FEIBA NF may be misleading and should be avoided due to the risk of DIC caused by overdose.
In cases of inadequate response to treatment with FEIBA NF, it is recommended to determine the platelet count, as an adequate number of functional platelets is necessary for the efficacy of FEIBA NF.
Do not exceed single doses of 100 units/kg body weight and a daily dose of 200 units/kg body weight. Patients receiving more than 100 units/kg body weight should be monitored for signs of DIC and (or) acute coronary syndrome. High doses of FEIBA NF should only be administered for the period necessary to stop the bleeding.
If significant changes in blood pressure, heart rate, respiratory disturbances, chest pain, or coughing occur, discontinue the administration of the medicine immediately and initiate appropriate diagnostic and therapeutic measures.
Laboratory results indicating DIC include decreased fibrinogen levels, decreased platelet count, and the presence of fibrin degradation products (FDP).
Administration of FEIBA NF to patients with inhibitors may result in an initial anamnestic increase in inhibitor levels. Under the influence of further administration of FEIBA NF, the inhibitor level may decrease over time. Clinical and literature data indicate that the efficacy of FEIBA NF is not reduced.
During administration of FEIBA NF, patients with hemophilia and inhibitors or acquired inhibitors of clotting factors may experience an increased risk of bleeding and thrombosis.
See also "Warnings and precautions" section.
Detailed information on this medicine is available in the Summary of Product Characteristics on the website of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterTakeda Manufacturing Austria AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Feiba NfDosage form: Powder, 500 IU = 500 IU FEIBAActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription requiredDosage form: Powder, 1000 IU (1000 IU FEIBA), 50 IU/mlActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription requiredDosage form: Powder, 500 IU (500 IU FEIBA), 50 IU/mlActive substance: factor VIII inhibitor bypassing activityManufacturer: Takeda Manufacturing Austria AGPrescription required
Alternatives to Feiba Nf in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Feiba Nf in Spain
Online doctors for Feiba Nf
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Feiba Nf – subject to medical assessment and local rules.














