
TAPTIQOM 15 micrograms/ml + 5 mg/ml eye drops solution in single-dose containers

How to use TAPTIQOM 15 micrograms/ml + 5 mg/ml eye drops solution in single-dose containers
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Taptiqom 15 micrograms/ml + 5 mg/ml eye drops, solution in single-dose container
Tafluprost / Timolol
Read the package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Taptiqom and what is it used for
- What you need to know before you use Taptiqom
- How to use Taptiqom
- Possible side effects
- Storage of Taptiqom
- Contents of the pack and other information
1. What is Taptiqom and what is it used for
What kind of medicine is it and how does it work?
Taptiqom eye drops, solution, contain tafluprost and timolol. Tafluprost is a medicine belonging to a group called prostaglandin analogues, and timolol belongs to a group of medicines called beta-blockers. Tafluprost and timolol work together to reduce eye pressure. Taptiqom is used when the eye pressure is too high.
What is your medicine used for?
Taptiqom is used to treat a type of glaucoma called open-angle glaucoma, a condition also known as ocular hypertension in adults. Both conditions are related to an increase in pressure in the eye and may occasionally affect your vision.
2. What you need to know before you use Taptiqom
Do not use Taptiqom:
- if you are allergic to tafluprost, timolol, beta-blockers, or any of the other ingredients of this medicine (listed in section 6).
- if you have or have had respiratory problems such as asthma, severe chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, or prolonged coughing).
- if you have a slow heart rate, heart failure, or heart rhythm disorders (irregular heartbeat).
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Taptiqom.
Before using this medicine, tell your doctor if you have or have had:
- heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, or low blood pressure
- heart rhythm disorders, such as slow heartbeat
- respiratory problems, asthma, or chronic obstructive pulmonary disease
- poor blood circulation (such as Raynaud's disease or Raynaud's syndrome)
- diabetes, as timolol may mask the signs and symptoms of hypoglycemia (low blood sugar levels)
- hyperthyroidism, as timolol may mask the signs and symptoms of thyroid disease
- any allergy or anaphylactic reaction
- severe myasthenia (a severe disease that causes muscle weakness)
- other eye diseases, such as corneal disease (the transparent tissue that covers the front of the eye) or a disease that requires eye surgery.
Tell your doctor if you have:
- kidney problems
- liver problems
Be awarethat Taptiqom may have the following effects, and some may be permanent:
- Taptiqom may increase the length, thickness, color, or density of your eyelashes and cause unusual hair growth on your eyelids.
- Taptiqom may cause darkening of the skin around the eyes. Wipe away any excess solution from the skin. This will reduce the risk of skin darkening.
- Taptiqom may change the color of your iris (the colored part of your eye). If you use Taptiqom in one eye only, the color of the treated eye may become permanently different from the color of the other eye.
- Taptiqom may cause hair growth in areas where the solution comes into repeated contact with the skin surface.
If you are going to have surgery, tell your doctor that you are using Taptiqom, as timolol may affect the effects of some medicines used during anesthesia.
Children and adolescents
Taptiqom is not recommended for children and adolescents under 18 years of age due to the lack of data on safety and efficacy in this age group.
Taking Taptiqom with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Taptiqom may affect, or be affected by, other medicines you are taking.
In particular, tell your doctor if you are using/taking or plan to use/take:
- other eye drops for glaucoma treatment
- medicines to lower blood pressure
- heart medicines
- medicines for diabetes treatment
- quinidine (used to treat heart problems and some types of malaria)
- antidepressants known as fluoxetine or paroxetine
If you use other medicines in the eye, wait at least 5 minutes between the administration of Taptiqom and the other medicine.
Contact lenses
Remove your contact lenses before administering the drops and wait at least 15 minutes before putting them back in.
Pregnancy, breastfeeding, and fertility
If you are a woman who can become pregnant, you must use an effective contraceptive method during treatment with Taptiqom. Do not use Taptiqom if you are pregnant. Do not use Taptiqom if you are breastfeeding. Talk to your doctor.
Driving and using machines
Some side effects associated with Taptiqom, such as blurred vision, may affect your ability to drive vehicles or operate machinery. Do not drive or operate machinery until you feel well and your vision is clear.
Taptiqom contains phosphate buffer
This medicine contains approximately 0.04 mg of phosphates per drop, equivalent to 1.3 mg/ml. If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use Taptiqom
Follow the instructions for administration of this medicine exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one drop of Taptiqom per day in the affected eye(s). Do not instill more drops or more often than indicated by your doctor. If you do, Taptiqom may lose its effectiveness. Use Taptiqom in both eyes only if your doctor has told you to do so. Discard the opened container and any remaining contents immediately after use.
For use only as eye drops. Do not ingest.
Do notlet the single-dose container touch the eye or the surrounding area. This could damage the eye. It could also become contaminated with bacteria that could cause eye infections, which could lead to eye damage, including vision loss. To avoid possible contamination of the single-dose container, avoid letting the tip touch any surface.
Instructions for use:
When opening a new bag:
Do not use the single-dose container if the bag is broken. Open the bag by tearing along the dotted line. Write the date you opened the bag in the space provided on the bag.
Each time you use Taptiqom:
- Wash your hands.
- Remove the strip of containers from the bag.
- Detach a single-dose container from the strip.
- Put the rest of the strip back in the bag and fold the edge to close it.
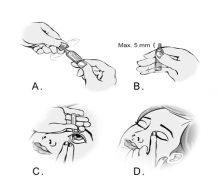
- To open the container, break the tab by twisting it (Figure A).
- Hold the container with your thumb and index finger. Make sure the tip of the container does not protrude more than 5 mm from the edge of your index finger (Figure B).
- Tilt your head back or lie down. Place your hand on your forehead. Your index finger should be aligned with your eyebrow or rest on the bridge of your nose. Look up. Press down the upper eyelid with your other hand. Do not let any part of the container touch the eye or the surrounding area. Gently press the container to release one drop into the space between the eyelid and the eye (Figure C).
- Close your eye and press the inner corner of your eye with your finger for about two minutes. This helps prevent the drop from draining through the tear duct (Figure D).
- Wipe away any excess solution from the skin around the eye.
If the drop falls outside the eye,try again.
If your doctor has told you to apply drops in both eyes, repeat steps 7 to 9 in the other eye. The contents of one single-dose container are sufficient for both eyes. Discard the opened container and any remaining contents immediately after use.
If you use other medicines in the eye,wait at least 5 minutes between the administration of Taptiqom and the other medicine.
If you use more Taptiqom than you should, you may feel dizzy or have a headache, heart problems, or breathing difficulties. If necessary, consult a doctor.
If you swallow the medicine accidentally, consult a doctor.
You can also call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use Taptiqom, put one drop in as soon as you remember and go back to your normal routine. However, if it is almost time for your next dose, skip the missed dose. Do not take a double dose to make up for forgotten doses.
Do not stop using Taptiqom without talking to your doctor. If you stop treatment with Taptiqom, the pressure in your eye will increase again. This could lead to permanent damage to your eye. If you have any other questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Most side effects are not serious.
Normally, you will be able to continue using the drops except if the effects are severe. If you are unsure, talk to a doctor or pharmacist.
The known side effects of using Taptiqom are:
Common side effects
The following effects may affect up to 1 in 10 people:
Eyelid disorders
Itching of the eyes. Irritation of the eyes. Eye pain. Redness of the eyes. Changes in length, thickness, and density of the eyelashes. Feeling of a foreign body in the eye. Discoloration of the eyelashes. Sensitivity to light. Blurred vision.
Uncommon side effects
The following effects may affect up to 1 in 100 people:
Nervous system disorders
Headache.
Eyelid disorders
Dry eye. Redness of the eyelids. Small areas of inflammation on the surface of the eye. Watery eyes. Swelling of the eyelids. Tired eyes. Inflammation of the eyelids. Inflammation inside the eye. Eye discomfort. Allergic eye reaction. Eye inflammation. Abnormal sensation in the eye.
The following additional side effects have been observed with the medicines that make up Taptiqom (tafluprost and timolol) and may therefore occur when using Taptiqom:
The following side effects have been observed with tafluprost:
Eyelid disorders
Reduced ability of the eye to see details. Change in iris color (may be permanent). Change in skin color around the eyes. Inflammation of the membranes on the surface of the eye. Eye discharge. Pigmentation of the membranes on the surface of the eye. Follicles on the membranes on the surface of the eye. Sunken eye. Iritis/uveitis (inflammation of the colored part of the eye). Macular edema/cystoid macular edema (inflammation of the retina inside the eye leading to vision impairment).
Skin disorders
Unusual hair growth on the eyelids.
Respiratory system effects
Worsening of asthma, respiratory failure.
The following side effects have been observed with timolol:
Immune system disorders
Allergic reactions, including inflammation under the skin, hives, and rashes. Sudden and potentially life-threatening allergic reaction. Itching.
Metabolism and nutrition disorders
Hypoglycemia (low blood sugar levels).
Psychiatric disorders
Depression. Sleep disorders. Nightmares. Memory loss. Nervousness. Hallucinations.
Nervous system disorders
Dizziness. Weakness. Unusual sensations (such as tingling and pinching). Increased signs and symptoms of myasthenia gravis (muscle disorder). Stroke. Reduced blood flow to the brain.
Eyelid disorders
Inflammation of the cornea. Reduced corneal sensitivity. Visual disturbances, including refractive changes (sometimes due to the abandonment of miotic therapy). Drooping of the upper eyelid. Double vision. Blurred vision and detachment of the layer under the retina, which contains blood vessels, after filtration surgery, which can cause vision disturbances. Corneal erosion.
Hearing disorders
Ringing in the ears (tinnitus).
Cardiac disorders
Slow heartbeat. Chest pain. Palpitations. Edema (fluid accumulation). Changes in heart rate or rhythm. Congestive heart failure (heart disease with difficulty breathing and swelling of feet and legs due to fluid accumulation). A type of heart rhythm disorder. Myocardial infarction. Heart failure.
Vascular disorders
Low blood pressure. Claudication. Raynaud's phenomenon, cold hands and feet.
Respiratory disorders
Contraction of airways in the lungs (especially in patients with pre-existing disease). Difficulty breathing. Cough.
Gastrointestinal disorders
Nausea. Indigestion. Diarrhea. Dry mouth. Taste disturbances. Abdominal pain. Vomiting.
Skin disorders
Hair loss. Skin rash with a white, scaly appearance (rash like psoriasis) or worsening of psoriasis. Skin rash.
Musculoskeletal and connective tissue disorders
Muscle pain not caused by exercise. Joint pain.
Reproductive system and breast disorders
Peyronie's disease (which can cause curvature of the penis). Sexual dysfunction. Decreased libido.
General disorders
Muscle weakness/fatigue. Thirst.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Taptiqom
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the single-dose container, the bag, and the carton after "EXP". The expiry date is the last day of the month shown.
Store unopened aluminum bags in a refrigerator (between 2°C and 8°C). Do not open the bag until you are about to start using the eye drops, as unused containers from an opened bag must be discarded 28 days after first opening the bag.
After opening the aluminum bag:
- Store single-dose containers in the original aluminum bag to protect them from light.
- Store below 25°C.
- Discard single-dose containers 28 days after first opening the aluminum bag.
- Discard opened single-dose containers with remaining solution immediately after use.
Medicines should not be disposed of via wastewater or household waste. Return containers and medicines you no longer need to the SIGRE collection point at your pharmacy. If you are unsure, ask your pharmacist how to dispose of containers and medicines you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition of Taptiqom
- The active ingredients are tafluprost and timolol. 1 ml of solution contains 15 micrograms of tafluprost and 5 mg of timolol.
- The other components are glycerol, disodium phosphate dodecahydrate, disodium edetate, polysorbate 80, hydrochloric acid or sodium hydroxide (for pH adjustment), and water for injectable preparations.
Appearance of the product and container contents
Taptiqom is a clear and colorless liquid (solution) presented in single-dose plastic containers containing 0.3 ml of solution each. The single-dose containers are contained, ten by ten, in a bag. Taptiqom is supplied in containers with 30 or 90 single-dose containers.
Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7th floor
28045 – Madrid
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Taptiqom:Germany, Austria, Belgium, Bulgaria, Cyprus, Croatia, Denmark, Slovakia, Slovenia, Spain, Estonia, Finland, France, Greece, Netherlands, Hungary, Ireland, Iceland, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Portugal, United Kingdom (Northern Ireland), Czech Republic, Romania, Sweden
Loyada:Italy
Date of last revision of this leaflet:June 2022
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price26.09 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAPTIQOM 15 micrograms/ml + 5 mg/ml eye drops solution in single-dose containersDosage form: EYEDROP, 10 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: EYEDROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/ml + 5 mg/mlActive substance: timolol, combinationsManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for TAPTIQOM 15 micrograms/ml + 5 mg/ml eye drops solution in single-dose containers
Discuss questions about TAPTIQOM 15 micrograms/ml + 5 mg/ml eye drops solution in single-dose containers, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















