
ROSALGIN PRONTO 140 mg SOLUCIÓN VAGINAL

Cómo usar ROSALGIN PRONTO 140 mg SOLUCIÓN VAGINAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para la usuaria
Rosalgin pronto 140 mg solución vaginal
hidrocloruro de bencidamina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su farmacéutico.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si necesita consejo o más información, consulte a su farmacéutico.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
- Debe consultar a un médico si empeora o si no mejora después de 5 días.
Contenido del prospecto
- Qué es Rosalgin pronto y para qué se utiliza
- Qué necesita saber antes de empezar a usar Rosalgin pronto
- Cómo usar Rosalgin pronto
- Posibles efectos adversos
- Conservación de Rosalgin pronto
- Contenido del envase e información adicional
1. Qué es Rosalgin pronto y para qué se utiliza
Pertenece a un grupo de medicamentos denominados antiinflamatorios para administración vaginal.
Este medicamento está indicado para el alivio local y temporal del picor y escozor, complementario al tratamiento de vaginitis, en adultos.
Debe consultar a un médico si empeora o si no mejora después de 5 días.
2. Qué necesita saber antes de empezar a usar Rosalgin pronto
No use Rosalgin pronto
- Si es alérgica a la bencidamina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o su farmacéutico antes de empezar a usar Rosalgin pronto.
- Este medicamento se utiliza sólo por vía vaginal.
- Una vez aplicada la solución, se recomienda lavar las manos con abundante agua, ya que se han descrito casos de reacciones alérgicas fotosensibles tras la exposición de las manos a la luz solar, sin haber eliminado previamente el producto (ver sección 4).
- En el caso de sensibilidad o irritación, o de quemazón o prurito persistente debe suspender el tratamiento y consultar con su médico (ver sección 4).
- Si observa aumento de flujo vaginal o cambios en su aspecto u olor o sangrado, debe consultar a su médico.
Otros medicamentos yRosalgin pronto
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No se conocen interacciones con otros medicamentos.
Uso de Rosalgin pronto con alimentos, bebidas y alcohol
No procede por ser un medicamento de uso vaginal.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe utilizar Rosalgin pronto durante el embarazo a menos que sea claramente necesario y su médico se lo aconseje. Si necesita tratamiento, debe utilizar la dosis más baja durante el menor tiempo posible.
Conducción y uso de máquinas
La utilización de este medicamento no altera la capacidad de conducir ni la utilización de máquinas, puesto que la absorción es prácticamente nula o insignificante.
Rosalgin pronto contiene cloruro de benzalconio, etanol y fragancias que contienen alérgenos
Este medicamento contiene 28 mg de cloruro de benzalconio por cada frasco (140 ml). El cloruro de benzalconio puede provocar irritación local.
Este medicamento contiene 107,8 mg de etanol por cada frasco (140 ml). Puede causar sensación de ardor en piel lesionada.
Este medicamento contiene fragancias con alcohol bencílico, benzoato de bencilo, cinamato de bencilo, salicilato de bencilo, citral, citronelol, eugenol, farnesol, geraniol, linalol y d-limoneno.
El alcohol bencílico, benzoato de bencilo, cinamato de bencilo, salicilato de bencilo, citral, citronelol, eugenol, farnesol, geraniol, linalol y d-limoneno pueden provocar reacciones alérgicas.
3. Cómo usar Rosalgin pronto
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
ESTE MEDICAMENTO SE UTILIZA SÓLO POR VÍA VAGINAL.
La dosis recomendada es de 1 ó 2 irrigaciones vaginales al día durante 5 días.
Una vez aplicada la solución, lavar las manos con abundante agua.
En caso de usar otros medicamentos por vía vaginal, Rosalgin pronto se debe administrar al principio, con el fin de evitar la eliminación del otro medicamento.
Puede utilizarse a temperatura ambiente o introduciendo el frasco cerrado durante algunos minutos en agua templada.
Para utilizar el aplicador unidosis siga los siguientes pasos:
 1º Para abrir el frasco mantener fijo el anillo superior, según la figura, y girar el capuchón hasta romper el precinto.
1º Para abrir el frasco mantener fijo el anillo superior, según la figura, y girar el capuchón hasta romper el precinto.
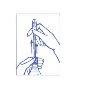 2º Extraer la cánula hasta notar el choque con la parte superior. Sólo la extracción completa de la cánula permitirá la salida del líquido.
2º Extraer la cánula hasta notar el choque con la parte superior. Sólo la extracción completa de la cánula permitirá la salida del líquido.
3º Introducir delicadamente la cánula en la vagina y comprimir el frasco hasta vaciarlo.
El vaciado puede ser gradual. Una válvula incorporada impide el reflujo de la solución al frasco.
Para conseguir una actividad terapéutica plena, el líquido debe ser mantenido en la vagina durante algunos minutos.
Si usa más Rosalgin pronto del que debe
No se han observado casos de sobredosis con Rosalgin pronto utilizado por vía vaginal.
En caso de ingestión oral accidental de hidrocloruro de bencidamina puede no tener síntomas o puede sentir náuseas, vómitos, dolor abdominal, mareo, alucinaciones visuales o irritabilidad, debilidad muscular, temblor, somnolencia, dolor de cabeza, visión borrosa, hipertensión arterial, entre otras. No existe ningún antídoto específico, sin embargo, suele remitir espontáneamente una vez que el fármaco es eliminado. Puede ser necesario tratamiento sintomático.
Si ingiere accidentalmente algo de su medicamento, contacte inmediatamente con su médico o farmacéutico para que le aconseje. También puede llamar al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el nombre del medicamento y la cantidad ingerida.
Si olvidó usar Rosalgin pronto
No se aplique una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, Rosalgin pronto puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos muy raros (menos de 1 de cada 10.000 personas):
- Sensibilidad de la piel a la luz del sol (que causa erupción o quemadura solar), quemazón, picor en la zona de aplicación.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Rosalgin pronto
No requiere condiciones especiales de conservación.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Rosalgin pronto
- El principio activo es bencidamina (como hidrocloruro de bencidamina). Cada frasco contiene 140 mg de hidrocloruro de bencidamina.
- Los demás componentes (excipientes) son: cloruro de benzalconio, edetato de disodio, etanol, polisorbato 20, aceite de rosa (contiene: alcohol bencílico, benzoato de bencilo, cinamato de bencilo, salicilato de bencilo, citral, citronelol, eugenol, farnesol, geraniol, linalol y d-limoneno) y agua purificada.
Aspecto del producto y contenido del envase
Rosalgin pronto se presenta en forma de solución vaginal. La solución es transparente incolora y está envasada en frascos de polietileno con 140 ml de solución.
Titular de la autorización de comercialización
ANGELINI PHARMA ESPAÑA, S.L.
c/ Antonio Machado, 78-80
3ª planta, módulo A-Edificio Australia
08840 Viladecans, Barcelona (España)
Responsable de la fabricación
A.C.R.A.F. SpA
Via Vecchia del Pinocchio, 22
60131 Ancona (Italia)
Fecha de la última revisión de este prospecto:Julio 2025.
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ROSALGIN PRONTO 140 mg SOLUCIÓN VAGINALForma farmacéutica: LIQUIDO VAGINAL, 500 mg bencidamina hidrocloruroPrincipio activo: benzydamineFabricante: Angelini Pharma Espana S.L.No requiere recetaForma farmacéutica: COMPRIMIDO, 20 mgPrincipio activo: fructus agni castiFabricante: Bionorica SeNo requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 7,5 mg/mlPrincipio activo: AtosibanFabricante: Altan Pharmaceuticals SaRequiere receta
Médicos online para ROSALGIN PRONTO 140 mg SOLUCIÓN VAGINAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ROSALGIN PRONTO 140 mg SOLUCIÓN VAGINAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






