
POMBILITI 105 MG POLVO PARA CONCENTRADO PARA SOLUCION PARA PERFUSION

Cómo usar POMBILITI 105 MG POLVO PARA CONCENTRADO PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Pombiliti 105 mg polvo para concentrado para solución para perfusión
cipaglucosidasa alfa
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a recibir este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Pombiliti y para qué se utiliza
- Qué necesita saber antes de empezar a recibir Pombiliti
- Cómo se administra Pombiliti
- Posibles efectos adversos
- Conservación de Pombiliti
- Contenido del envase e información adicional
1. Qué es Pombiliti y para qué se utiliza
Pombiliti es un tipo de “terapia de sustitución enzimática” (TSE) que está indicado para adultos con enfermedad de Pompe de inicio tardío. Contiene el principio activo llamado “cipaglucosidasa alfa”.
Para qué se utiliza
Pombiliti se utiliza siempre junto con otro medicamento llamado miglustat 65 mg cápsulas duras. Es muy importante que usted también lea el prospecto de miglustat 65 mg cápsulas duras.
Si tiene cualquier duda sobre estos medicamentos, consulte a su médico o farmacéutico.
Cómo actúa Pombiliti
Las personas con la enfermedad de Pompe tienen niveles bajos de una enzima llamada α-glucosidasa ácida (GAA). Esta enzima ayuda a regular los niveles de glucógeno (un tipo de hidrato de carbono) en el organismo.
En la enfermedad de Pompe, se acumulan grandes cantidades de glucógeno en los músculos de todo el cuerpo. Esto impide el correcto funcionamiento de los músculos, por ejemplo los que ayudan a caminar, los que facilitan la respiración en los pulmones y el músculo cardíaco.
Pombiliti entra en las células musculares que están afectadas por la enfermedad de Pompe. Una vez en el interior de las células, el medicamento actúa como la GAA, favoreciendo la descomposición del glucógeno y regulando sus niveles.
2. Qué necesita saber antes de empezar a recibir Pombiliti
No debe recibir Pombiliti
- Si alguna vez ha tenido reacciones de hipersensibilidad potencialmente mortales a lo siguiente:
- cipaglucosidasa alfa
- miglustat
- alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si una perfusión anterior tuvo que interrumpirse y no se pudo reanudar debido a reacciones de hipersensibilidad potencialmente mortales.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Pombiliti
Consulte a su médico o enfermero de inmediatosi alguna de estas situaciones es aplicable en su caso, o cree que podrían serlo, o si alguna vez ha tenido alguna de estas reacciones con otra terapia de sustitución enzimática (TSE):
- reacciones alérgicas, incluida la anafilaxia (una reacción alérgica grave) - ver la sección 4 del apartado “Posibles efectos adversos”, más abajo, para conocer los síntomas de las reacciones potencialmente mortales;
- reacción asociada a la perfusión mientras está recibiendo el medicamento o en las horas posteriores, ver sección 4 del apartado “Posibles efectos adversos”, más abajo, para conocer los síntomas de las reacciones potencialmente mortales.
Informe a su médico si tiene antecedentes de alguna enfermedad cardíaca o pulmonar. Estas enfermedades pueden empeorar durante o inmediatamente después de la perfusión de Pombiliti. Informe a su médico o enfermero de inmediato si sufre dificultad para respirar, tos, latidos rápidos o irregulares del corazón o cualquier otro efecto de estas enfermedades.
Informe también al médico si presenta hinchazón en las piernas o hinchazón generalizada del cuerpo, erupción cutánea grave u orina espumosa al eliminar líquidos. El médico decidirá si la perfusión de Pombiliti debe interrumpirse y le dará el tratamiento médico adecuado. Asimismo, el médico decidirá si puede seguir recibiendo Pombiliti.
Medicamentos previos al tratamiento
Es posible que su médico le administre otros medicamentos antes del tratamiento con Pombiliti, por ejemplo:
- antihistamínicos y corticoesteroides para prevenir o mitigar las reacciones asociadas a la perfusión;
- antipiréticos para reducir la fiebre.
Niños y adolescentes
Este medicamento no debe administrarse a pacientes menores de 18 años, porque se desconocen los efectos de Pombiliti junto con miglustat en este grupo de edad.
Otros medicamentos y Pombiliti
Informe a su médico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluidos los medicamentos adquiridos sin receta y los medicamentos a base de plantas.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, no debe recibir este medicamento, sino consultar a su médico o farmacéutico de inmediato antes de utilizarlo.
No hay datos sobre el uso de Pombiliti en combinación con miglustat durante el embarazo.
- Si está embarazada, no debe recibir Pombiliti y / o tomar miglustat 65 mg cápsulas duras. Informe a su médico de inmediato si se queda embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada. Puede haber riesgos para el bebé en gestación.
- Pombiliti en combinación con miglustat no se debe administrar a mujeres en periodo de lactancia. Se debe decidir si es necesario dejar la lactancia o dejar el tratamiento.
Anticoncepción y fertilidad
Las mujeres en edad fértil deben utilizar métodos anticonceptivos efectivos, durante y 4 semanas después de finalizar la administración de ambos medicamentos.
Conducción y uso de máquinas
Es posible que tenga mareos, somnolencia o presión arterial baja (hipotensión) después de recibir Pombiliti o los medicamentos previos al tratamiento. En tal caso, no conduzca ni utilice herramientas o máquinas.
Pombiliti contiene sodio
Este medicamento contiene 10,5 mg de sodio (componente principal de la sal de mesa/para cocinar) en cada vial. Esto equivale al 0,52 % de la ingesta diaria máxima de sodio recomendada para un adulto.
3. Cómo se administra Pombiliti
Pombiliti es administrado por un médico o enfermero. Se administra en forma de goteo en el interior de una vena. Esta forma de administración se denomina perfusión intravenosa.
Consulte a su médico si desea tratarse en casa. Este decidirá, tras la evaluación, si es seguro que reciba la perfusión de Pombiliti en su domicilio. Si experimenta algún efecto secundaria durante una perfusión de Pombiliti, es posible que el miembro del personal encargado de administrársela detenga la perfusión e inicie el tratamiento farmacológico que corresponda.
Pombiliti debe usarse en combinación con miglustat. Solo puede utilizar miglustat 65 mg cápsulas con cipaglucosidasa alfa. NOutilice miglustat 100 mg cápsulas (medicamento diferente). Con respecto a la dosis recomendada, siga las instrucciones de su médico y lea el prospecto de miglustat 65 mg cápsulas duras.
Qué cantidad de Pombiliti se administra
La cantidad de medicamento que recibirá se basa en su peso. La dosis recomendada es de 20 mg por cada kilogramo de peso corporal.
Cuándo se administra Pombiliti y durante cuánto tiempo
- Recibirá tratamiento con Pombiliti una vez cada dos semanas. Miglustat 65 mg cápsulas se toma el mismo día de la administración de Pombiliti. Consulte el prospecto de miglustat 65 mg cápsulas duras para obtener información sobre cómo tomar miglustat.
- La perfusión de cipaglucosidasa alfa debe comenzar 1 hora después de haber tomado miglustat 65 mg cápsulas duras.
- En caso de demora, el inicio de la perfusión no debe exceder las 3 horas desde la toma de miglustat.
- La perfusión de cipaglucosidasa alfa dura unas 4 horas.
Figura 1. Desarrollo cronológico de las dosis
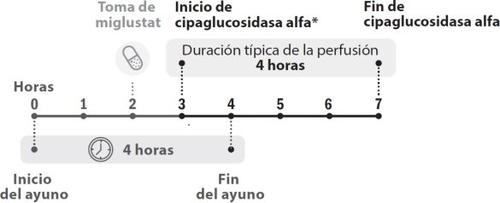
- La perfusión de cipaglucosidasa alfa debe iniciarse 1 hora después de haber tomado las cápsulas de miglustat. En caso de retraso en la perfusión, el inicio de esta no debe exceder las 3 horas desde la toma de miglustat.
Cambio desde otra terapia de sustitución enzimática (TSE)
Si actualmente está recibiendo otra TSE:
- Su médico le indicará cuándo debe dejar la otra TSE antes de iniciar Pombiliti.
- Informe a su médico de cuándo recibió la última dosis.
Si recibe más Pombiliti del que debe
Si tiene dificultad para respirar, siente hinchazón o inflamación, o nota el corazón acelerado, puede que le hayan administrado demasiado Pombiliti; informe a su médico de inmediato. El exceso de la velocidad de perfusión de Pombiliti podría provocar síntomas debidos a un exceso de líquido en el organismo, por ejemplo dificultad para respirar, frecuencia cardiaca alta o hinchazón generalizada en todo el cuerpo.
Si olvidó su dosis de Pombiliti
Si se ha saltado una perfusión, póngase en contacto con su médico o enfermero lo antes posible, para fijar una cita y que le puedan administrar Pombiliti en combinación con miglustat 24 horas después de la última toma de miglustat.
Si interrumpe el tratamiento con Pombiliti
Hable con su médico si desea interrumpir el tratamiento con Pombiliti. Los síntomas de su enfermedad quizá empeoren si interrumpe el tratamiento.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Pombiliti se utiliza junto con miglustat y cualquiera de estos medicamentos puede producir efectos adversos. Los efectos adversos se han observado principalmente en los pacientes durante la perfusión de Pombiliti (reacciones asociadas a la perfusión) o poco después. Informe a su médico de inmediato si sufre una reacción asociada a la perfusión o una reacción alérgica. Algunas de estas reacciones pueden ser graves y potencialmente mortales. Es posible que su médico le administre medicamentos antes de la perfusión para prevenir estas reacciones.
Reacciones asociadas a la perfusión
La mayoría de las reacciones asociadas a la perfusión son leves o moderadas. Los síntomas de una reacción asociada a la perfusión son, entre otros, dificultad para respirar, hinchazón, fiebre, escalofríos, mareos, enrojecimiento y picor en la piel y sarpullido.
Reacciones alérgicas
Las reacciones alérgicas pueden dar lugar a síntomas tales como sarpullido en cualquier parte del cuerpo, hinchazón de los ojos, dificultad para respirar prolongada, tos, hinchazón de los labios, la lengua o la garganta, picor en la piel y urticaria.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Dolor de cabeza
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- Tos
- Enrojecimiento repentino de la cara, el cuello o la parte superior del pecho
- Dolor en el pecho
- Sarpullido, picor
- Aumento de la presión arterial
- Sudoración
- Hinchazón abdominal
- Flatulencia o gases
- Diarrea, heces sueltas
- Vómitos
- Náuseas
- Fiebre o escalofríos
- Urticaria
- Hinchazón o dolor en la zona de inserción de la aguja
- Calambres, dolor o debilidad musculares
- Temblores en una o varias partes del cuerpo
- Aumento de la sudoración
- Dolor
- Alteración del sentido del gusto
- Sensación de cansancio constante o de sueño
- Dificultad para respirar
Poco frecuentes(pueden afectar a hasta 1 de cada 100 personas)
- Respiración dificultosa y que provoca tos, silbidos (sibilancias) al respirar y sensación de falta de aire (asma)
- Reacción alérgica
- Hinchazón de las manos, los pies, los tobillos, las piernas
- Hinchazón de la cara
- Indigestión
- Dolor de estómago
- Sensación de cansancio constante
- Dolor o irritación de garganta
- Contracciones dolorosas y anormales de la garganta
- Irritación de la boca
- Dolor en la boca o molestias en la parte de atrás de la boca
- Dolor en las mejillas, las encías, los labios, la barbilla
- Pérdida de fuerza y energía, sensación de debilidad
- Malestar, sensación general de letargo
- Sensación de ardor
- Rasguños o lesiones en la piel
- Alteraciones de la temperatura corporal
- Disminución de un tipo de glóbulo blanco (detectada en los análisis)
- Somnolencia
- Mareos
- Dolor en las articulaciones
- Dolor en la zona entre la cadera y las costillas
- Fatiga muscular
- Mayor rigidez de los músculos
- Incapacidad para mantener el equilibrio
- Presión arterial baja
- Sensación de estar a punto del desmayo
- Dolor en uno o ambos lados de la cabeza, dolor punzante, aura, dolor en los ojos, sensibilidad a la luz (migraña)
- Manchas en la piel
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Pombiliti
Su médico, farmacéutico o enfermero es el responsable de conservar este medicamento y de desechar correctamente los viales abiertos. Esta información está destinada únicamente a profesionales sanitarios.
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y en la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Viales sin abrir: Conservar en nevera (entre 2 ºC y 8 ºC). Conservar el vial en el embalaje exterior para protegerlo de la luz.
Después de la dilución, se recomienda un uso inmediato. Sin embargo, la conservación de la bolsa de perfusión intravenosa con Pombiliti se ha demostrado durante 6 horas a una temperatura entre 20 ºC y 25ºC y durante 24 horas a una temperatura entre 2 ºC y 8 ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Pombiliti
El principio activo es cipaglucosidasa alfa. Un vial contiene 105 mg de cipaglucosidasa alfa. Después de la reconstitución, la solución contenida en el vial contiene 15 mg de cipaglucosidasa alfa por mililitro. Se recomienda una concentración final de cipaglucosidasa alfa diluida en la bolsa de perfusión intravenosa de 0,5 mg/ml a 4 mg/ml.
Los demás componentes son:
- Citrato de socio dihidratado (E331)
- Ácido cítrico monohidratado (E330)
- Manitol (E421)
- Polisorbato 80 (E433)
Aspecto del producto y contenido del envase
Pombiliti es un polvo blanco a ligeramente amarillo. Después de la reconstitución, es una solución de transparente a opalescente, entre incolora y ligeramente amarilla, sin partículas extrañas y prácticamente libre de partículas de color blanco a translúcidas. La solución reconstituida se debe diluir posteriormente en una bolsa de perfusión intravenosa.
Pombiliti es un polvo para concentrado para solución para perfusión en un vial.
Envases de 1, 10 o 25 viales
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Amicus Therapeutics Europe Limited
Block 1, Blanchardstown Corporate Park
Ballycoolin Road
Blanchardstown, Dublin
D15 AKK1
Irlanda
Tel.: +353 (0) 1 588 0836
Fax: +353 (0) 1 588 6851
Correo electrónico: [email protected]
Responsable de la fabricación
Manufacturing Packaging Farmaca (MPF) B.V.
Neptunus 12, Heerenveen, 8448CN, Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Amicus Therapeutics Europe Limited Tél/Tel: (+32) 0800 89172 e-mail: [email protected] | Lietuva Amicus Therapeutics Europe Limited Tel: (+370) 8800 33167 El. paštas: [email protected] |
| Luxembourg/Luxemburg Amicus Therapeutics Europe Limited Tél/Tel: (+352) 800 27003 e-mail: [email protected] |
Ceská republika Amicus Therapeutics Europe Limited Tel.: (+420) 800 142 207 e-mail: [email protected] | Magyarország Amicus Therapeutics Europe Limited Tel.: (+36) 06 800 21202 e-mail: [email protected] |
Danmark Amicus Therapeutics Europe Limited Tlf.: (+45) 80 253 262 e-mail: [email protected] | Malta Amicus Therapeutics Europe Limited Tel: (+356) 800 62674 e-mail: [email protected] |
Deutschland Amicus Therapeutics GmbH Tel: (+49) 0800 000 2038 E-Mail: [email protected] | Nederland Amicus Therapeutics BV Tel: (+31) 20 235 8510/(+31) 0800 022 8399 e-mail: [email protected] |
Eesti Amicus Therapeutics Europe Limited Tel: (+372) 800 0111 911 e-post: [email protected] | Norge Amicus Therapeutics Europe Limited Tlf: (+47) 800 13837 e-post: [email protected] |
Ελλáδα Amicus Therapeutics Europe Limited Τηλ: (+30) 00800 126 169 e-mail: [email protected] | Österreich Amicus Therapeutics Europe Limited Tel: (+43) 0800 909 639 e-mail: [email protected] |
España Amicus Therapeutics S.L.U. Tel: (+34) 900 941 616 e-mail: [email protected] | Polska Amicus Therapeutics Europe Limited Tel.: (+48) 0080 012 15475 e-mail: [email protected] |
France Amicus Therapeutics SAS Tél: (+33) 0 800 906 788 e-mail: [email protected] | Portugal Amicus Therapeutics Europe Limited Tel: (+351) 800 812 531 e-mail: [email protected] |
Hrvatska Amicus Therapeutics Europe Limited Tel: (+358) 0800 222 452 e-pošta: [email protected] | Ireland Amicus Therapeutics Europe Limited Tel: (+353) 1800 936 230 e-mail: [email protected] |
România Amicus Therapeutics Europe Limited Tel.: (+40) 0808 034 288 e-mail: [email protected] | Slovenija Amicus Therapeutics Europe Limited Tel.: (+386) 0800 81794 e-pošta: [email protected] |
Ísland Amicus Therapeutics Europe Limited Sími: (+354) 800 7634 Netfang: [email protected] | Slovenská republika Amicus Therapeutics Europe Limited Tel: (+421) 0800 002 437 e-mail: [email protected] |
Italia Amicus Therapeutics S.r.l. Tel: (+39) 800 795 572 e-mail: [email protected] | Suomi/Finland Amicus Therapeutics Europe Limited Puh/Tel: (+358) 0800 917 780 sähköposti/e-mail: [email protected] |
Κúπρος Amicus Therapeutics Europe Limited Τηλ: (+357) 800 97595 e-mail: [email protected] | Sverige Amicus Therapeutics Europe Limited Tfn: (+46) 020 795 493 e-post: [email protected] |
Latvija Amicus Therapeutics Europe Limited Tel: (+371) 800 05391 e-pasts: [email protected] | United Kingdom (Northern Ireland) Amicus Therapeutics, UK Limited Tel: (+44) 08 0823 46864 e-mail: [email protected] |
Fecha de la última revisión de este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
Esta información está destinada únicamente a profesionales sanitarios:
Instrucciones de uso: reconstitución, dilución y administración
Pombiliti se debe reconstituir con agua para preparaciones inyectables y, a continuación, diluir en una solución de cloruro de sodio 9 mg/ml (0,9 %) para preparaciones inyectables y administrar mediante perfusión intravenosa. La reconstitución y dilución debe realizarse de acuerdo con las normas de buena práctica clínica, especialmente en cuanto al respeto de la asepsia.
Dado que este medicamento es una proteína, es posible que se formen partículas en la solución reconstituida y en la bolsa de perfusión diluida final. Por tanto, se debe utilizar un filtro en línea de 0,2 micrómetros de baja unión a proteínas para la administración. Se ha demostrado que el uso de un filtro en línea de 0,2 micrómetros elimina las partículas visibles y no provoca una pérdida aparente de proteínas ni de actividad.
Determine el número de viales que se han de reconstituir en función de la pauta posológica de cada paciente (mg/kg) y saque los viales necesarios de la nevera para que alcancen la temperatura ambiente (unos 30 minutos). Cada vial de Pombiliti es de un solo uso.
Use una técnica aséptica.
Reconstitución
Reconstituya los 105 mg por vial de Pombiliti en 7,2 ml de agua para preparaciones inyectables utilizando una jeringa con un diámetro de aguja no superior a 18 G. Añada el agua para preparaciones inyectables gota a gota por el lateral del vial y no directamente sobre el polvo liofilizado. Incline y vaya girando cada vial con cuidado. No invierta, remueva ni agite el vial. El volumen de extracción es una solución de transparente a opalescente, entre incolora y ligeramente amarilla, sin partículas extrañas y prácticamente libre de partículas de color blanco a translúcidas. Efectúe una inspección inmediata de los viales reconstituidos para verificar que no haya partículas ni alteración del color. Si en la inspección inmediata se observan partículas extrañas distintas a las descritas anteriormente o la solución reconstituida presenta una alteración del color, no la utilice. El pH de la solución reconstituida es de aproximadamente 6,0.
Después de la reconstitución, se recomienda diluir los viales de inmediato (ver a continuación).
Dilución
Tras la reconstitución descrita anteriormente, la solución reconstituida en el vial contiene 15 mg de cipaglucosidasa alfa por mililitro. El volumen reconstituido permite la extracción exacta de 7,0 ml (equivalente a 105 mg) de cada vial. La solución se debe diluir posteriormente de la siguiente manera: con una jeringa de diámetro de aguja no superior a 18 G, extraiga lentamente la solución reconstituida de cada vial, incluido el volumen inferior a 7,0 ml del vial parcial, hasta obtener la dosis del paciente. La concentración final recomendada de cipaglucosidasa alfa en las bolsas de perfusión está comprendida entre 0,5 mg/ml y 4 mg/ml. Extraiga el aire del interior de la bolsa de perfusión.
Asimismo, extraiga un volumen equivalente de solución inyectable de cloruro de sodio 9 mg/ml (0,9 %), que será sustituido por el volumen de Pombiliti reconstituido. Inyecte lentamente la solución reconstituida de Pombiliti directamente en la solución de cloruro de sodio 9 mg/ml (0,9 %) para preparaciones inyectables. Invierta o masajee con cuidado la bolsa de perfusión para mezclar la solución diluida. No sacuda ni agite excesivamente la bolsa de perfusión.
La solución final para perfusión debe administrarse lo antes posible tras la preparación.
La eliminación del medicamento no utilizado y de todos los materiales que hayan estado en contacto con él se realizará de acuerdo con la normativa local.
Administración
La perfusión de Pombiliti debe comenzar 1 hora después de haber tomado las cápsulas de miglustat. En caso de demora de la perfusión, su inicio no debe exceder las 3 horas desde la toma de miglustat. La pauta posológica recomendada de Pombiliti es de 20 mg/kg de peso corporal administrados cada dos semanas mediante perfusión intravenosa.
Las perfusiones se deben administrar de forma gradual. Se recomienda que la velocidad inicial de la perfusión sea de 1 mg/kg/h y se vaya aumentando gradualmente en 2 mg/kg/h cada 30 minutos, si no aparecen signos de RAP (reacciones asociadas a la perfusión), hasta que se alcance una velocidad máxima de 7 mg/kg/h.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a POMBILITI 105 MG POLVO PARA CONCENTRADO PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 100 UPrincipio activo: LaronidasaFabricante: Sanofi B.V.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 30 mg/mlPrincipio activo: Cerliponasa alfaFabricante: Biomarin International LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, DesconocidaPrincipio activo: ImiglucerasaFabricante: Sanofi B.V.Requiere receta
Médicos online para POMBILITI 105 MG POLVO PARA CONCENTRADO PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de POMBILITI 105 MG POLVO PARA CONCENTRADO PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















