
ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН

Запитайте лікаря про рецепт на ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН

Інструкція із застосування ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН
Введення
Опис: інформація для користувача
Opatanol 1мг/мл офтальмологічний розчин
олопатадин
Прочитайте уважно весь опис перед тим, як почати використовувати цейлікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь сумніви, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки для вас, і не давайте його іншим людям, навіть якщо вони мають相同ні симптоми, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це побічні ефекти, які не наведені в цьому описі. Див. розділ 4.
Зміст опису
- Що таке Opatanol і для чого він використовується
- Що потрібно знати перед тим, як почати використовувати Opatanol
- Як використовувати Opatanol
- Можливі побічні ефекти
- Збереження Opatanol
- Зміст упаковки та додаткова інформація
1. Що таке Opatanol і для чого він використовується
Opatanolпоказаний для лікування ознак і симптомів сезонного алергійного кон'юнктивіту.
Алергічний кон'юнктивіт.Деякі фактори (називані алергенами) як пилок, домашній пил або шерсть тварин можуть викликати алергічні реакції, які призводять до свербіння і червоного кольору, а також запалення поверхні очей.
Opatanol належить до групи лікарських засобів, які використовуютьсядля лікування алергічних захворювань очей. Він діє шляхом зменшення інтенсивності алергічної реакції.
2. Що потрібно знати перед тим, як почати використовувати Opatanol
Не використовуйтеOpatanol
- Якщо ви алергічні(гіпersenситивні) до олопатадину або до одного з інших компонентів цього лікарського засобу (перелічених у розділі 6).
- Не використовуйте Opatanol, якщо ви перебуваєте в період лактації.
Попередження та застереження
Проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як почати використовувати Opatanol.
Ви повинні видалити контактні лінзи з очей перед тим, як використовувати Opatanol.
Діти
Не використовуйте Opatanol у дітей молодших 3 років. Не застосовуйте цей лікарський засіб дітям молодших 3 років, оскільки немає даних, які свідчать про те, що він безпечний і діє у дітей молодших 3 років.
Інші лікарські засоби та Opatanol
Повідомте вашого лікаря або фармацевта, якщо ви використовуєте, нещодавно використовували або можете використовувати будь-який інший лікарський засіб.
Якщо ви використовуєте інший офтальмологічний розчин або мазь, чекайте принаймні 5 хвилин між застосуванням кожного лікарського засобу. Офтальмологічні мазі повинні застосовуватися в останню чергу.
Вагітність, лактація та фертильність
Якщо ви вагітні або перебуваєте в період лактації, вважаєте, що можете бути вагітною або маєте намір завагітніти, проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як використовувати цей лікарський засіб.
Не використовуйте Opatanol, якщо ви перебуваєте в період лактації, проконсультуйтеся з вашим лікарем перед тим, як використовувати цей лікарський засіб.
Водіння транспортних засобів та використання машин
Відразу після застосування Opatanol ви можете відчувати, що ваш зір розмитий. Не водьте транспортні засоби та не використовуйте машини до тих пір, поки цей ефект не зникне.
Opatanolмістить бензалконій хлорид
Цей лікарський засіб містить 0,5 мг бензалконію хлориду в кожних 5 мл, що еквівалентно 0,1 мг/мл.
Консервант Opatanol, бензалконій хлорид, може бути поглинений м'якими контактними лінзами та може змінити колір контактних лінз. Видаліть контактні лінзи перед тим, як використовувати цей лікарський засіб, і чекайте 15 хвилин перед тим, як знову їх вдягнути.
Бензалконій хлорид може викликати очне подразнення, особливо якщо ви страждаєте на сухе око або інші захворювання рогівки (прозорої оболонки передньої частини ока). Проконсультуйтеся з вашим лікарем, якщо ви відчуваєте якусь незвичайну відчуття, свербіння або біль в оці після використання цього лікарського засобу.
Opatanol містить дигідрофосфат дісоду дodecahydrat
Цей лікарський засіб містить 16,72 мг фосфатів (у 63,05 мг дигідрофосфату дісоду дodecahydrat) в кожному флаконі по 5 мл, що еквівалентно 3,34 мг/мл.
Якщо ви страждаєте на важке пошкодження рогівки (прозорої оболонки передньої частини ока), лікування фосфатами в рідких випадках може викликати розмитість зору через накопичення кальцію.
3. Як використовувати Opatanol
Слідуйте точно інструкціям щодо застосування цього лікарського засобу, вказаним вашим лікарем або фармацевтом. У разі сумнівів проконсультуйтеся знову з вашим лікарем або фармацевтом.
Рекомендована доза становить одну краплю в одне око або в обидва очі, двічі на день - вранці та ввечері.
Використовуйте цю кількість, якщо тільки ваш лікар не дав вам інших інструкцій. Opatanol повинен застосовуватися тільки в обидва очі, якщо ваш лікар так вказав. Продовжуйте лікування протягом періоду часу, вказаного вашим лікарем.
Opatanol повинен використовуватися тільки як краплі для очей.
ДЛЯ БІЛЬШОЇ ІНФОРМАЦІЇ ПЕРЕЙДІТЬ НА ЗАДНЮ ЧАСТИНУ ПРОСПЕКТУ
Переверніть частину проспекта.
Як використовувати Opatanol(продовження)
1 2
Кількість для застосування
Перейдіть на передню частину проспекта
- Візьміть флакон Opatanol і поставтеся перед дзеркалом.
- Вимийте руки.
- Візьміть флакон і відкрутите кришку.
- Після видалення кришки необхідно видалити пластикову ободку з упаковки перед тим, як використовувати.
- Тримайте флакон, зверху донизу, між великим і вказівним пальцями.
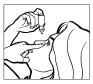
- Наклоніть голову назад. Ніжно відокремте повіку від ока пальцем, поки не утвориться мішечок, у який повинна потрапити крапля (Фігура 1).
- Приведіть наконечник флакона до ока. Вам може знадобитися дзеркало.
- Не торкайтеся ока, повіки, прилеглих ділянок чи інших поверхонь наконечником флакона, оскільки краплі, які залишилися в флаконі, можуть бути інфіковані.
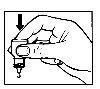
- Ніжно натисніть на основу флакона, щоб одна крапля Opatanol потрапила кожен раз.
- Не стискайте флакон, він розроблений так, щоб легкий тиск на основу був достатнім (Фігура 2).
- Якщо ви застосовуєте краплі в обидва очі, повторіть попередні пункти для іншого ока.
- Закрутите кришку на флакон негайно після використання продукту.
Якщо крапля потрапила поза око, спробуйте знову.
Якщо ви використовуєте більше Opatanol, ніж потрібно
Ви можете видалити його, промивши очі теплою водою. Не застосовуйте більше крапель до наступного застосування.
Якщо ви забули використовувати Opatanol
Застосуйте одну краплю, як тільки ви про це вспомните, і продовжуйте наступну дозу вашого звичайного режиму. Однак, якщо вже майже час наступної дози, не застосовуйте пропущену дозу і продовжуйте наступну дозу вашого звичайного режиму. Не застосовуйте подвійну дозу для компенсації пропущених доз.
Якщо ви припините лікування Opatanol
Не припиняйте використання цього лікарського засобу без попередньої консультації з вашим лікарем.
Якщо у вас є якісь інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
З Opatanol спостерігалися наступні побічні ефекти:
Часті (можуть впливати до 1 особи з 10)
Ефекти в оці
Біль в оці, подразнення в оці, сухе око, ненормальне відчуття в оці, дискомфорт в оці.
Загальні ефекти
Головний біль, втома, суха ніс, поганий смак у роті.
Рідкі (можуть впливати до 1 особи з 100)
Ефекти в оці
Розмитість зору, зниження або ненормальне зору, порушення рогівки, запалення поверхні ока з або без пошкодження поверхні, інфекція або запалення кон'юнктиви, виділення з ока, чутливість до світла, збільшення виробництва сльоз, свербіння в оці, червоний колір в оці, ненормальність повіки, свербіння, червоний колір, набряк або криста повіки.
Загальні ефекти
Зниження або ненормальне сприйняття стимулів, головокружіння, насморк, суха шкіра, запалення шкіри.
Частота невідома (не може бути оцінена з наявних даних)
Ефекти в оці
Набряк в оці, набряк рогівки, зміна розміру зіниці.
Загальні ефекти
Зважність дихання, збільшення алергічних симптомів, набряк обличчя, оніміння, загальна слабкість, нудота, блювота, інфекція синусів, червоний колір і свербіння шкіри.
У рідких випадках у деяких пацієнтів з важким пошкодженням прозорої оболонки передньої частини ока (рогівки) під час лікування фосфатами з'явилися темні плями на рогівці через накопичення кальцію.
Звіт про побічні ефекти
Якщо ви відчуваєте будь-який побічний ефект, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не наведені в цьому описі. Ви також можете повідомити про них безпосередньо через національну систему повідомлень, включену до додатка V. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Збереження Opatanol
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, вказаного на флаконі та упаковці після "CAD". Термін придатності - останній день місяця, який вказано.
Цьому лікарському засобу не потрібні спеціальні умови зберігання.
Щоб уникнути інфекцій, ви повинні видалити флакон через чотири тижні після першого відкриття та використовувати новий флакон. Запишіть дату відкриття в призначеному місці на етикетці кожного флакона та упаковки.
Лікарські засоби не повинні викидати в каналізацію чи сміття. Спитайте вашого фармацевта, як позбутися упаковок та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Opatanol
- Активний інгредієнт - олопатадин. Кожен мл розчину містить 1 мг олопатадину (у вигляді гідрохлориду).
- Інші компоненти - бензалконій хлорид, хлорид натрію, дигідрофосфат дісоду дodecahydrat (E339), хлоридна кислота (E507) та/або гідроксид натрію (E524) та очищена вода.
Вигляд продукту та вміст упаковки
Opatanol - прозора та безбарвна рідина (розчин), яка випускається в упаковці, що містить флакон по 5 мл або три пластикові флакони по 5 мл з кришкою.
Можливо, що тільки деякі розміри упаковок будуть випускатися.
Власник дозволу на маркетинг
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ірландія
Відповідальний за виробництво
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Іспанія
Novartis Manufacturing N.V.
Rijksweg 14
2870 Puurs-Sint-Amands
Бельгія
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Німеччина
Siegfried El Masnou, S.A.
Camil Fabra 58
El Masnou
08320 Barcelona
Іспанія
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Німеччина
Ви можете запитати більше інформації про цей лікарський засіб, звернувшись до місцевого представника власника дозволу на маркетинг.
Бельгія/Бельгія/Бельгія Novartis Pharma N.V. Тел./Телефон: +32 2 246 16 11 | Литва SIA Novartis Baltics Lietuvos filialas Телефон: +370 5 269 16 50 |
Болгарія Novartis Bulgaria EOOD Телефон: +359 2 489 98 28 | Люксембург/Люксембург Novartis Pharma N.V. Тел./Телефон: +32 2 246 16 11 |
Чехія Novartis s.r.o. Телефон: +420 225 775 111 | Угорщина Novartis Hungária Kft. Телефон: +36 1 457 65 00 |
Данія Novartis Healthcare A/S Телефон: +45 39 16 84 00 | Мальта Novartis Pharma Services Inc. Телефон: +356 2122 2872 |
Німеччина Novartis Pharma GmbH Телефон: +49 911 273 0 | Нідерланди Novartis Pharma B.V. Телефон: +31 88 04 52 111 |
Естонія SIA Novartis Baltics Eesti filiaal Телефон: +372 66 30 810 | Норвегія Novartis Norge AS Телефон: +47 23 05 20 00 |
Греція Novartis (Hellas) A.E.B.E. Телефон: +30 210 281 17 12 | Австрія Novartis Pharma GmbH Телефон: +43 1 86 6570 |
Іспанія Novartis Farmacéutica, S.A. Телефон: +34 93 306 42 00 | Польща Novartis Poland Sp. z o.o. Телефон: +48 22 375 4888 |
Франція Novartis Pharma S.A.S. Телефон: +33 1 55 47 66 00 | Португалія Novartis Farma - Produtos Farmacêuticos, S.A. Телефон: +351 21 000 8600 |
Хорватія Novartis Hrvatska d.o.o. Телефон: +385 1 6274 220 | Румунія Novartis Pharma Services Romania SRL Телефон: +40 21 31299 01 |
Ірландія Novartis Ireland Limited Телефон: +353 1 260 12 55 | Словенія Novartis Pharma Services Inc. Телефон: +386 1 300 75 50 |
Ісландія Vistor hf. Телефон: +354 535 7000 | Словаччина Novartis Slovakia s.r.o. Телефон: + 421 2 5542 5439 |
Італія Novartis Farma S.p.A. Телефон: +39 02 96 54 1 | Фінляндія Novartis Finland Oy Телефон: +358 (0)10 6133 200 |
Кіпр Novartis Pharma Services Inc. Телефон: +357 22 690 690 | Швеція Novartis Sverige AB Телефон: +46 8 732 32 00 |
Латвія SIA Novartis Baltics Телефон: +371 67 887 070 |
Дата останнього перегляду цього опису:
Інші джерела інформації
Детальна інформація про цей лікарський засіб доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu

Скільки коштує ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН в Іспанії у 2026 році?
ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН коштує в середньому 7.74 євро у січень, 2026 році. Ціна може змінюватися залежно від регіону, аптеки та наявності рецепта. Рекомендуємо перевіряти актуальну вартість у місцевих аптеках або через онлайн-сервіси.
- Країна реєстрації
- Середня ціна в аптеках7.74 EUR
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИНФорма випуску: ОЧНІ КРАПЛІ, 1 мг/млДіючі речовини: olopatadineВиробник: Tiedra Farmaceutica S.L.Потрібен рецептФорма випуску: ОЧНІ КРАПЛІ, 1 мг/млДіючі речовини: olopatadineВиробник: Qualix Pharma S.L.Потрібен рецептФорма випуску: ОЧНІ КРАПЛІ, 1 мг/мл мг/млДіючі речовини: olopatadineВиробник: Juta Pharma GmbhПотрібен рецепт
Аналоги ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН у Polónia
Аналог ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН у Ukraine
Лікарі онлайн щодо ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ОПАТАНОЛ 1 мг/мл КРАПЛІ ОЧНІ, РОЗЧИН – за рішенням лікаря та згідно з місцевими правилами.














